Answered step by step
Verified Expert Solution
Question
1 Approved Answer
urgent 3. The accompanying data give experimental values of the capillary rise of various liquids: Table 3.1. Experimental values of canillary rise for water hanzane
urgent 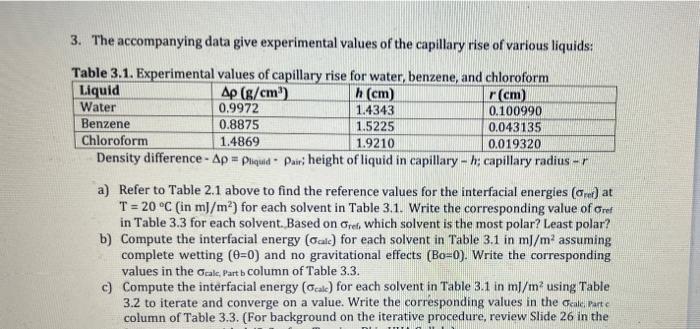
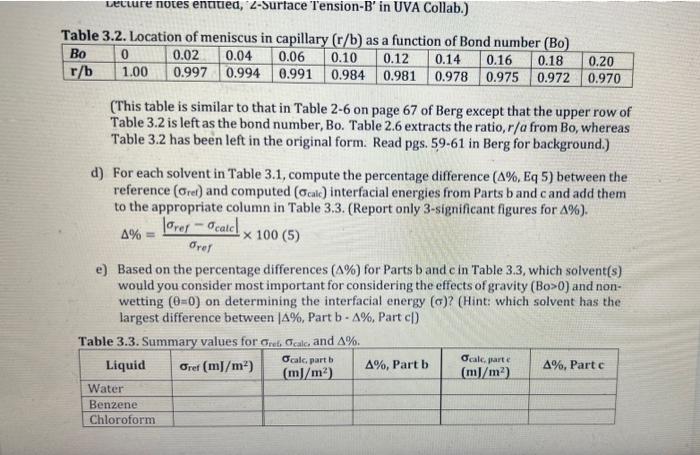
3. The accompanying data give experimental values of the capillary rise of various liquids: Table 3.1. Experimental values of canillary rise for water hanzane and fhloroform Density difference =liquidairi height of liquid in capillary h; capillary radius r a) Refer to Table 2.1 above to find the reference values for the interfacial energies (ref) at T=20C (in mJ/m2 ) for each solvent in Table 3.1. Write the corresponding value of ref in Table 3.3 for each solvent. Based on ref, which solvent is the most polar? Least polar? b) Compute the interfacial energy (alc) for each solvent in Table 3.1inmJ/m2 assuming complete wetting (=0) and no gravitational effects (Bo=0). Write the corresponding values in the Gak, Part b column of Table 3.3. c) Compute the interfacial energy ( alk) for each solvent in Table 3.1inm/m2 using Table 3.2 to iterate and converge on a value. Write the corresponding values in the Galk partc column of Table 3.3. (For background on the iterative procedure, review Slide 26 in the Lecture notes entided, 'L-Surface 'rension-B' in UVA Collab.) able 3.2. Location of meniscus in capillary (r/b) as a function of Rond numher (Rn) (This table is similar to that in Table 2-6 on page 67 of Berg except that the upper row of Table 3.2 is left as the bond number, Bo. Table 2.6 extracts the ratio, r/a from Bo, whereas Table 3.2 has been left in the original form. Read pgs. 59-61 in Berg for background.) d) For each solvent in Table 3.1, compute the percentage difference (%,Eq5) between the reference (ret) and computed (calc) interfacial energies from Parts b and c and add them to the appropriate column in Table 3.3. (Report only 3-significant figures for % ). %=refrefcalc100(5) e) Based on the percentage differences (%) for Parts b and c in Table 3.3, which solvent(s) would you consider most important for considering the effects of gravity (B>0) and nonwetting (=0) on determining the interfacial energy () ? (Hint: which solvent has the largest difference between %, Part b%, Part c|) 3. The accompanying data give experimental values of the capillary rise of various liquids: Table 3.1. Experimental values of canillary rise for water hanzane and fhloroform Density difference =liquidairi height of liquid in capillary h; capillary radius r a) Refer to Table 2.1 above to find the reference values for the interfacial energies (ref) at T=20C (in mJ/m2 ) for each solvent in Table 3.1. Write the corresponding value of ref in Table 3.3 for each solvent. Based on ref, which solvent is the most polar? Least polar? b) Compute the interfacial energy (alc) for each solvent in Table 3.1inmJ/m2 assuming complete wetting (=0) and no gravitational effects (Bo=0). Write the corresponding values in the Gak, Part b column of Table 3.3. c) Compute the interfacial energy ( alk) for each solvent in Table 3.1inm/m2 using Table 3.2 to iterate and converge on a value. Write the corresponding values in the Galk partc column of Table 3.3. (For background on the iterative procedure, review Slide 26 in the Lecture notes entided, 'L-Surface 'rension-B' in UVA Collab.) able 3.2. Location of meniscus in capillary (r/b) as a function of Rond numher (Rn) (This table is similar to that in Table 2-6 on page 67 of Berg except that the upper row of Table 3.2 is left as the bond number, Bo. Table 2.6 extracts the ratio, r/a from Bo, whereas Table 3.2 has been left in the original form. Read pgs. 59-61 in Berg for background.) d) For each solvent in Table 3.1, compute the percentage difference (%,Eq5) between the reference (ret) and computed (calc) interfacial energies from Parts b and c and add them to the appropriate column in Table 3.3. (Report only 3-significant figures for % ). %=refrefcalc100(5) e) Based on the percentage differences (%) for Parts b and c in Table 3.3, which solvent(s) would you consider most important for considering the effects of gravity (B>0) and nonwetting (=0) on determining the interfacial energy () ? (Hint: which solvent has the largest difference between %, Part b%, Part c|) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


