Answered step by step
Verified Expert Solution
Question
1 Approved Answer
urgent help please!!! Check my work Enter your answer in the provided box. From the data below, calculate the total heat in J) needed to
urgent help please!!! 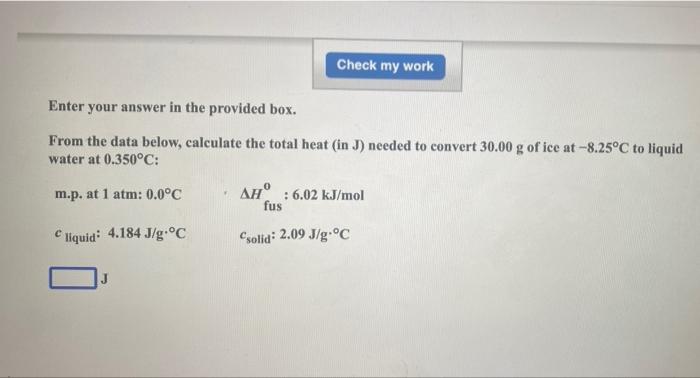
Check my work Enter your answer in the provided box. From the data below, calculate the total heat in J) needed to convert 30.00 g of ice at -8.25C to liquid water at 0.350C: m.p. at 1 atm: 0.0C AH : 6.02 kJ/mol fus liquid: 4.184 J/g.C Csolid: 2.09 J/g.C 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


