Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Urgently needed are problem 3 and 6. The rest you can send afterwards. *Kindly show balanced chemical equations, calculations and the graph* Problem Three -

Urgently needed are problem 3 and 6. The rest you can send afterwards.
*Kindly show balanced chemical equations, calculations and the graph*
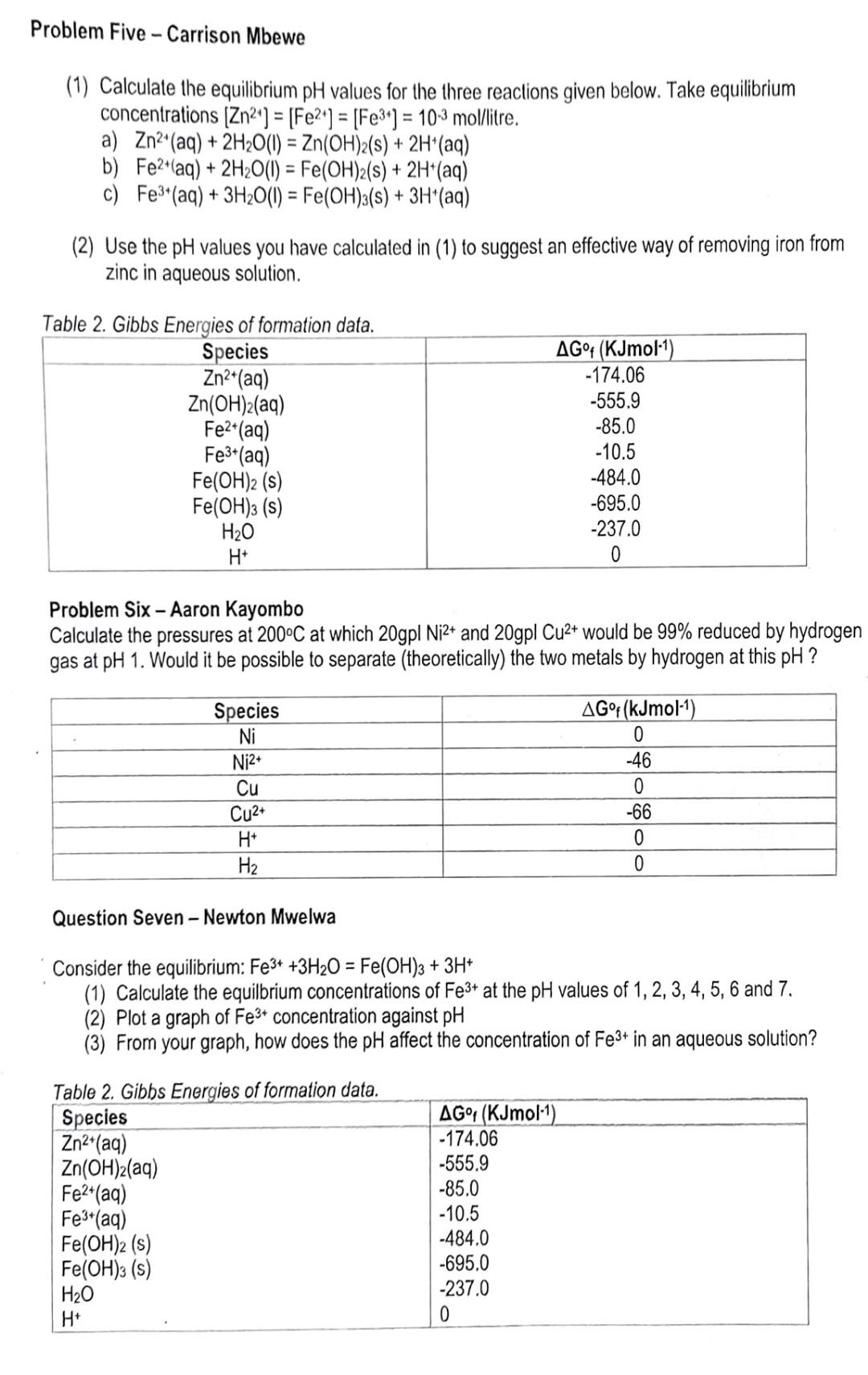
Problem Three - Audrick Mwaba (R=8.314JK1mol,1Faraday=96500coulombsmol1,nX=2.303log10X,273K=0C) Please use graph paper. lgnoring dissolved species, draw an EhpH diagram to represent the equilibrium among magnetite, siderite, metallic iron and water, at a partial pressure of CO2 of 102,1 atmosphere total pressure and 25C. Indicate metastable equilibria (if any) by a dashed line. From the diagram answer Yes or No to the following questions: (i) Can iron and magnetite co-exist? (ii) Can water and siderite co-exist? (iii) Can oxygen and siderite co-exist? (iv) Can hydrogen and magnetite co-exist? Question Four - Ricky Mainda Calculate the pressure at 25C at which 1MCa2+(aq) and 1MZnO22(aq) would be 99% reduced by hydrogen at pH 14. Would it be possible to separate (theoretically) the two metals by hydrogen reduction at this pH ? Data: 1 Faraday =96500Cmol1 rroblem Five - Carrison Mbewe (1) Calculate the equilibrium pH values for the three reactions given below. Take equilibrium concentrations [Zn2+]=[Fe2+]=[Fe3+]=103mol//itre. a) Zn2+(aq)+2H2O(I)=Zn(OH)2(s)+2H+(aq) b) Fe2+(aq)+2H2O(I)=Fe(OH)2(s)+2H+(aq) c) Fe3+(aq)+3H2O(I)=Fe(OH)3(s)+3H+(aq) (2) Use the pH values you have calculated in (1) to suggest an effective way of removing iron from zinc in aqueous solution. Tahlo 2 Cihha Eunuriana af frumatian Nato Problem Six - Aaron Kayombo Calculate the pressures at 200C at which 20gplNi2+ and 20gplCu2+ would be 99% reduced by hydrogen gas at pH 1. Would it be possible to separate (theoretically) the two metals by hydrogen at this pH ? Question Seven - Newton Mwelwa Consider the equilibrium: Fe3++3H2O=Fe(OH)3+3H+ (1) Calculate the equilbrium concentrations of Fe3+ at the pH values of 1, 2, 3, 4,5, 6 and 7. (2) Plot a graph of Fe3+ concentration against pH (3) From your graph, how does the pH affect the concentration of Fe3+ in an aqueous solution? Tahlo 2 Cihhe Enorsioe of fnrmation data Problem Three - Audrick Mwaba (R=8.314JK1mol,1Faraday=96500coulombsmol1,nX=2.303log10X,273K=0C) Please use graph paper. lgnoring dissolved species, draw an EhpH diagram to represent the equilibrium among magnetite, siderite, metallic iron and water, at a partial pressure of CO2 of 102,1 atmosphere total pressure and 25C. Indicate metastable equilibria (if any) by a dashed line. From the diagram answer Yes or No to the following questions: (i) Can iron and magnetite co-exist? (ii) Can water and siderite co-exist? (iii) Can oxygen and siderite co-exist? (iv) Can hydrogen and magnetite co-exist? Question Four - Ricky Mainda Calculate the pressure at 25C at which 1MCa2+(aq) and 1MZnO22(aq) would be 99% reduced by hydrogen at pH 14. Would it be possible to separate (theoretically) the two metals by hydrogen reduction at this pH ? Data: 1 Faraday =96500Cmol1 rroblem Five - Carrison Mbewe (1) Calculate the equilibrium pH values for the three reactions given below. Take equilibrium concentrations [Zn2+]=[Fe2+]=[Fe3+]=103mol//itre. a) Zn2+(aq)+2H2O(I)=Zn(OH)2(s)+2H+(aq) b) Fe2+(aq)+2H2O(I)=Fe(OH)2(s)+2H+(aq) c) Fe3+(aq)+3H2O(I)=Fe(OH)3(s)+3H+(aq) (2) Use the pH values you have calculated in (1) to suggest an effective way of removing iron from zinc in aqueous solution. Tahlo 2 Cihha Eunuriana af frumatian Nato Problem Six - Aaron Kayombo Calculate the pressures at 200C at which 20gplNi2+ and 20gplCu2+ would be 99% reduced by hydrogen gas at pH 1. Would it be possible to separate (theoretically) the two metals by hydrogen at this pH ? Question Seven - Newton Mwelwa Consider the equilibrium: Fe3++3H2O=Fe(OH)3+3H+ (1) Calculate the equilbrium concentrations of Fe3+ at the pH values of 1, 2, 3, 4,5, 6 and 7. (2) Plot a graph of Fe3+ concentration against pH (3) From your graph, how does the pH affect the concentration of Fe3+ in an aqueous solution? Tahlo 2 Cihhe Enorsioe of fnrmation dataStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


