Answered step by step
Verified Expert Solution
Question
1 Approved Answer
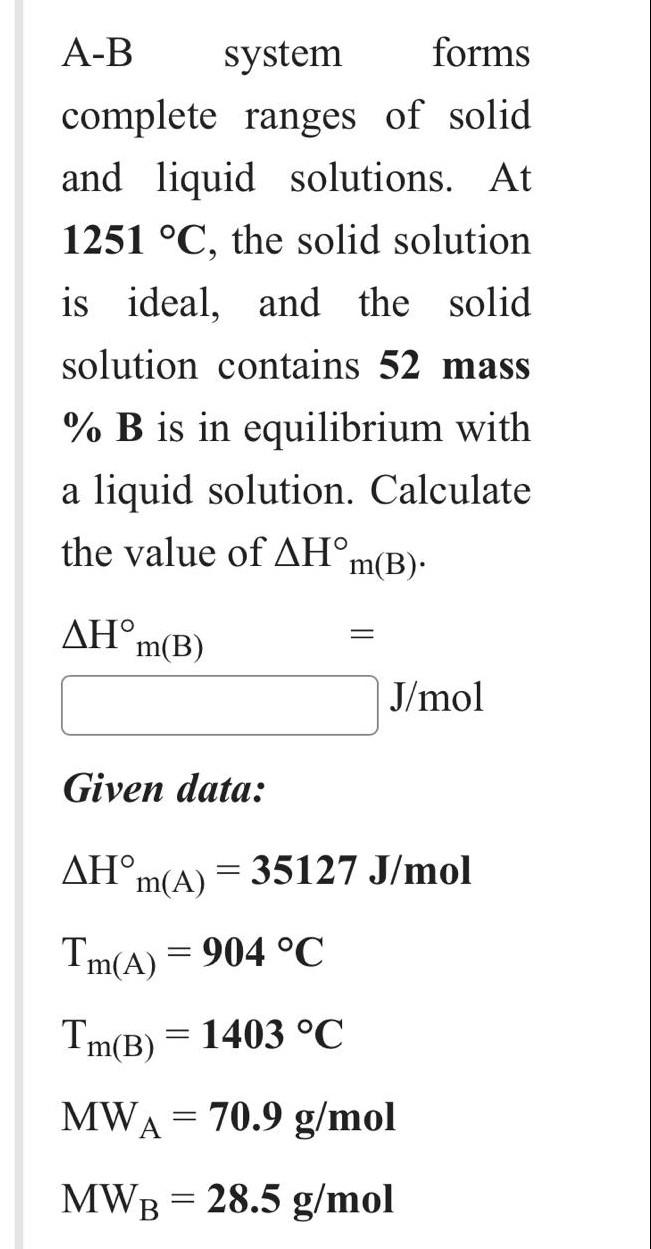
urgenttttttt please A-B system forms complete ranges of solid and liquid solutions. At 1251C, the solid solution is ideal, and the solid solution contains 52
urgenttttttt please
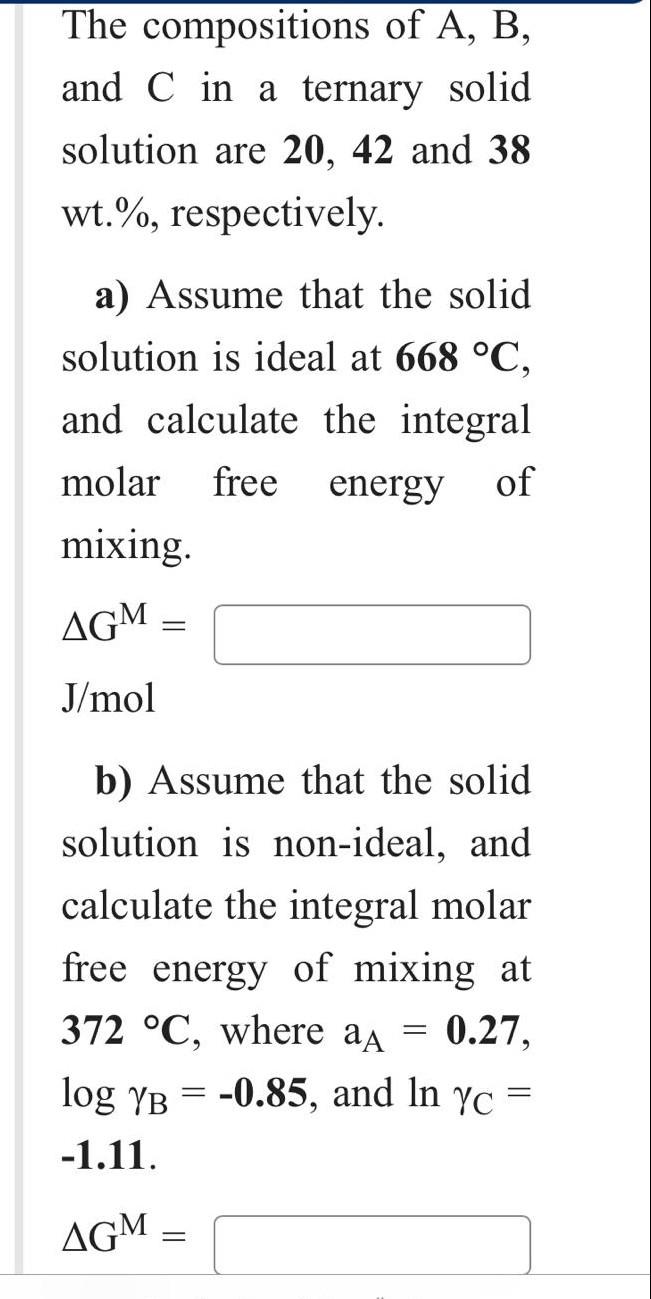
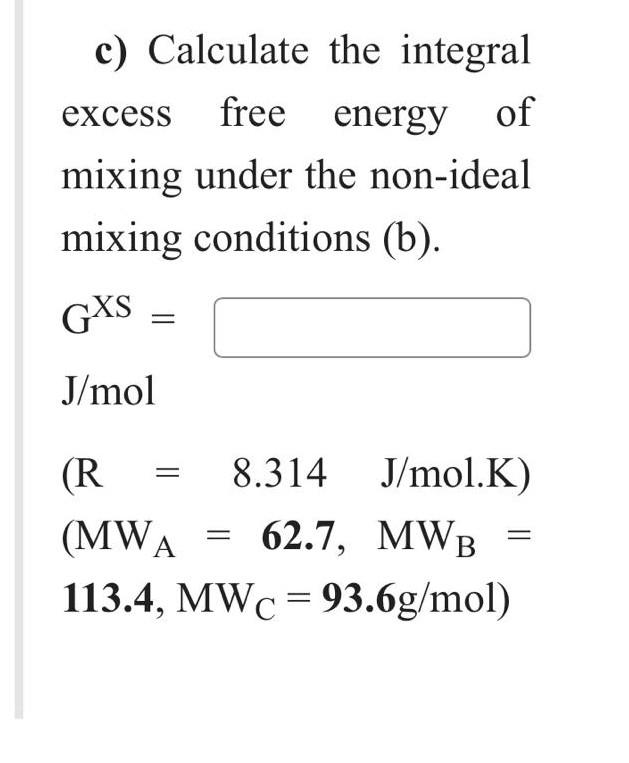
A-B system forms complete ranges of solid and liquid solutions. At 1251C, the solid solution is ideal, and the solid solution contains 52 mass %B is in equilibrium with a liquid solution. Calculate the value of Hm(B). Hm(B)= J/mol Given data: Hm(A)=35127J/molTm(A)=904CTm(B)=1403CMWA=70.9g/molMWB=28.5g/mol The compositions of A,B, and C in a ternary solid solution are 20, 42 and 38 wt.\%, respectively. a) Assume that the solid solution is ideal at 668C, and calculate the integral molar free energy of mixing. GM= J/mol b) Assume that the solid solution is non-ideal, and calculate the integral molar free energy of mixing at 372C, where aA=0.27, logB=0.85, and lnC= -1.11. GM= c) Calculate the integral excess free energy of mixing under the non-ideal mixing conditions (b). GXS= J/mol (R=8.314J/mol.K)(MWA=62.7,MWB=113.4,MWC=93.6g/mol) A-B system forms complete ranges of solid and liquid solutions. At 1251C, the solid solution is ideal, and the solid solution contains 52 mass %B is in equilibrium with a liquid solution. Calculate the value of Hm(B). Hm(B)= J/mol Given data: Hm(A)=35127J/molTm(A)=904CTm(B)=1403CMWA=70.9g/molMWB=28.5g/mol The compositions of A,B, and C in a ternary solid solution are 20, 42 and 38 wt.\%, respectively. a) Assume that the solid solution is ideal at 668C, and calculate the integral molar free energy of mixing. GM= J/mol b) Assume that the solid solution is non-ideal, and calculate the integral molar free energy of mixing at 372C, where aA=0.27, logB=0.85, and lnC= -1.11. GM= c) Calculate the integral excess free energy of mixing under the non-ideal mixing conditions (b). GXS= J/mol (R=8.314J/mol.K)(MWA=62.7,MWB=113.4,MWC=93.6g/mol)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


