Answered step by step
Verified Expert Solution
Question
1 Approved Answer
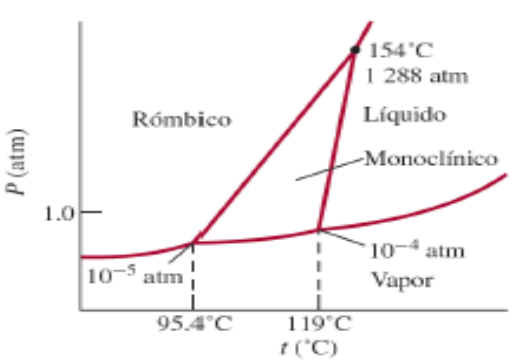
Usando el diagrama de fase del azufre determina: A ) Cu ntos puntos triples hay? B ) La monocl nica y la r mbica son
Usando el diagrama de fase del azufre determina:
ACuntos puntos triples hay?
B La monoclnica y la rmbica son dos formas alotrpicas del azufre. Cul es ms estable en condiciones atmosfricas
C Describe paso a paso qu sucede cuando el azufre a atm se calienta de a
D Si partiendo del punto crtico desciende la presin de hasta atm a T cte. Cules son las fases presentes y cules los grados de libertad de la lnea de equilibrio?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


