Answered step by step
Verified Expert Solution
Question
1 Approved Answer
use appropriate equations and diagram to solve Chemical Engineering Thermodynamics: An Ericsson cycle with air as the working fluid is operating between a low temperature
use appropriate equations and diagram to solve
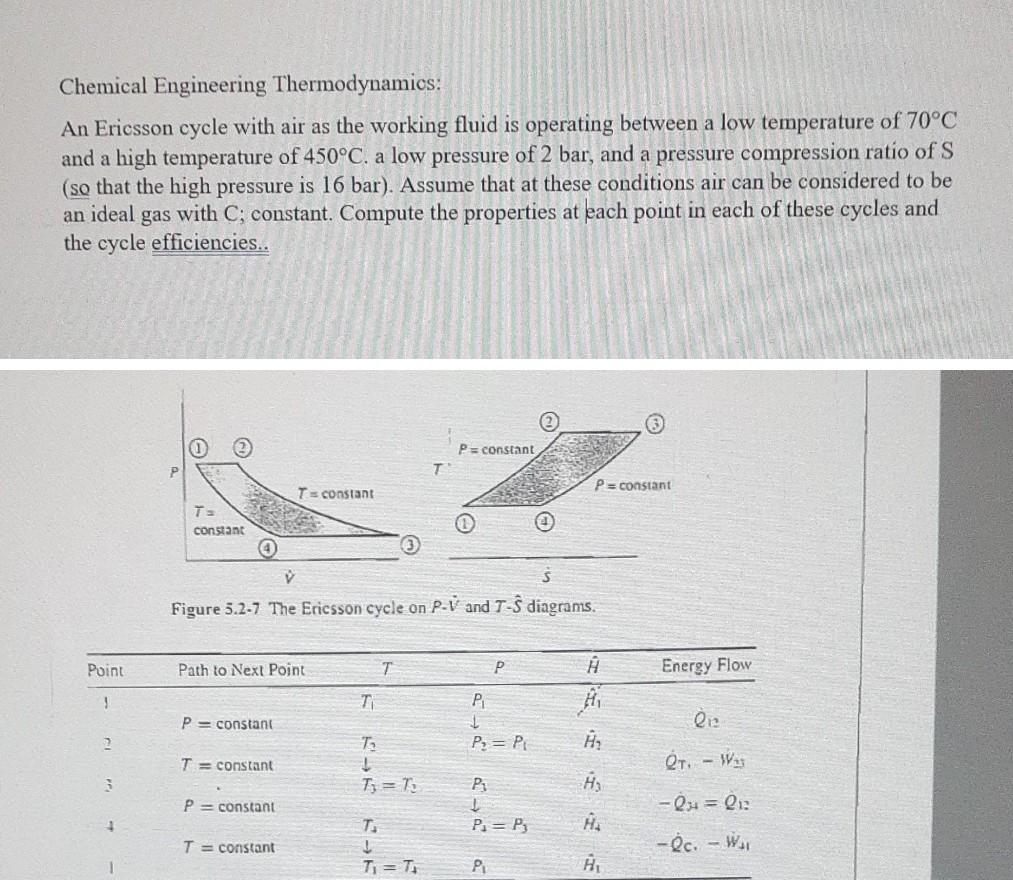
Chemical Engineering Thermodynamics: An Ericsson cycle with air as the working fluid is operating between a low temperature of 70C and a high temperature of 450C. a low pressure of 2 bar, and a pressure compression ratio of S (so that the high pressure is 16 bar). Assume that at these conditions air can be considered to be an ideal gas with C; constant. Compute the properties at each point in each of these cycles and the cycle efficiencies.. P = constant P=constant Ts constant TE constant V Figure 5.2-7 The Ericsson cycle on P-V and T- diagrams. Point Path to Next Point T P Energy Flow 1 TI PL P = constant # H Qiz 2 TO P = P T = constant OT - Was 3 Ti = 7: PA P = constant -Q1 = QI P = P3 HA T = constant T. 1 Ti = -Oc. - WAI P HStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


