Question
Use le Chatelier's principle to determine what happens to the following equilibrium at the given changes below. The answer options are: right, unchanged, left.: i.
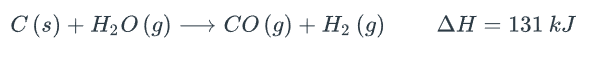
Use le Chatelier's principle to determine what happens to the following equilibrium at the given changes below. The answer options are: right, unchanged, left.:
i. When some hydrogen gas is removed, the equilibrium will be shifted towards..
ii. When some C (s) are added, the equilibrium will be shifted towards..
iii. When more H2O (g) is added, the equilibrium will be shifted towards...
iiii. As the pressure increases as the volume decreases (assuming the temperature is kept constant) the equilibrium will shift towards...
iiiii. When the gas mixture cools, the equilibrium will shift towards....
iiiiii. When a catalyst is added....
C(s) + H2O(g) + CO(g) + H2 (9) AH = 131 kJStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


