Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(Use the data from Turner et al. for all complexes except carbonate - see below) lg1(Ce(CO3)+)=5.33lg2(Ce(CO3)2)=9.24lg1(Eu(CO3)+)=5.75lg2(Eu(CO3)2)=10.11 Based on these calculations, estimate peq for the Ce(IV)/Ce
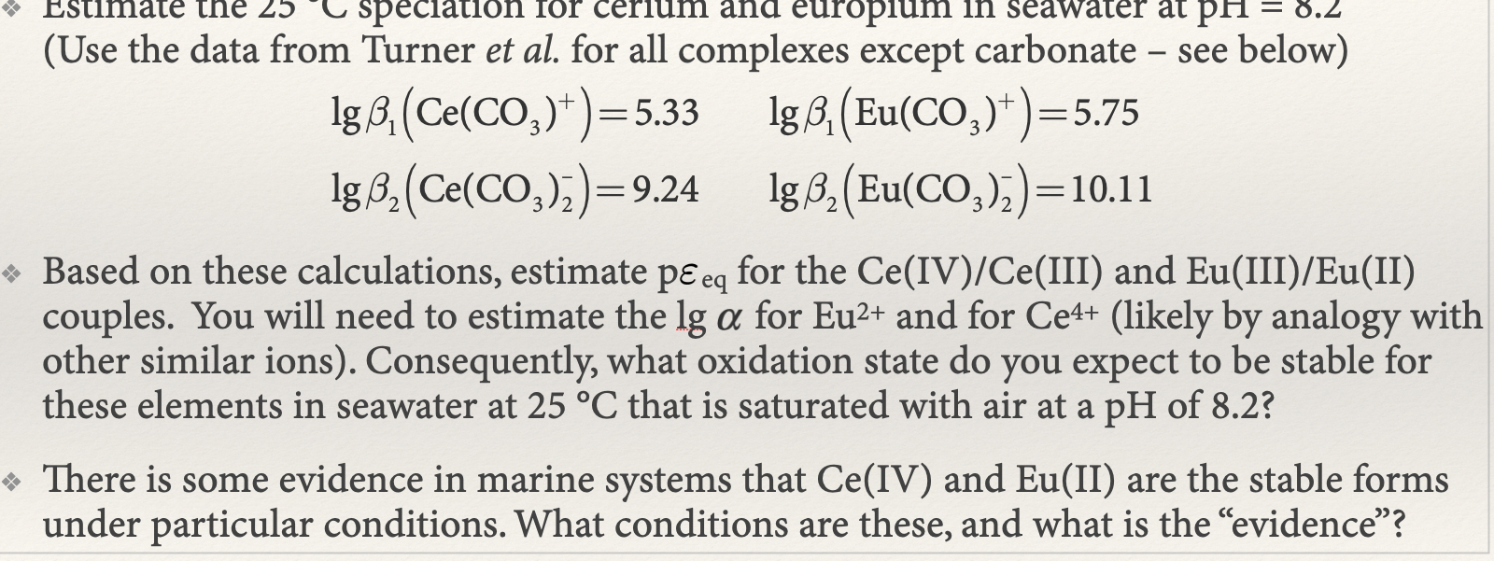 (Use the data from Turner et al. for all complexes except carbonate - see below) lg1(Ce(CO3)+)=5.33lg2(Ce(CO3)2)=9.24lg1(Eu(CO3)+)=5.75lg2(Eu(CO3)2)=10.11 Based on these calculations, estimate peq for the Ce(IV)/Ce (III) and Eu(III)/Eu(II) couples. You will need to estimate the lg for Eu2+ and for Ce4+ (likely by analogy with other similar ions). Consequently, what oxidation state do you expect to be stable for these elements in seawater at 25C that is saturated with air at a pH of 8.2 ? There is some evidence in marine systems that Ce(IV) and Eu(II) are the stable forms under particular conditions. What conditions are these, and what is the "evidence
(Use the data from Turner et al. for all complexes except carbonate - see below) lg1(Ce(CO3)+)=5.33lg2(Ce(CO3)2)=9.24lg1(Eu(CO3)+)=5.75lg2(Eu(CO3)2)=10.11 Based on these calculations, estimate peq for the Ce(IV)/Ce (III) and Eu(III)/Eu(II) couples. You will need to estimate the lg for Eu2+ and for Ce4+ (likely by analogy with other similar ions). Consequently, what oxidation state do you expect to be stable for these elements in seawater at 25C that is saturated with air at a pH of 8.2 ? There is some evidence in marine systems that Ce(IV) and Eu(II) are the stable forms under particular conditions. What conditions are these, and what is the "evidence Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


