Question
Use the equilibrium of o-nitrophenol in water below to answer the following questions. 17. What is the algebraic form of the equilibrium expression Ka in
Use the equilibrium of o-nitrophenol in water below to answer the following questions.
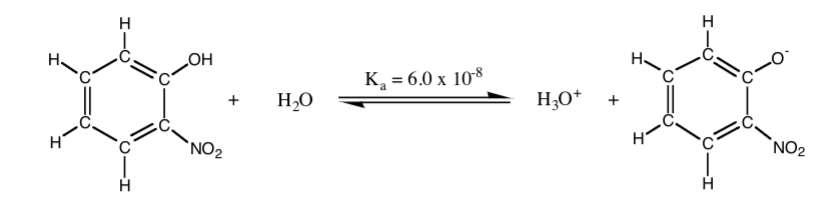
17. What is the algebraic form of the equilibrium expression Ka in the equilibrium above?
18. Given that the solubility of o-nitrophenol in water is 2.1 g/L, what will be the concentration in moles per liter (Molarity) of a saturated aqueous solution of o- nitrophenol, [C6H5NO3] ?
19. What will be the equilibrium concentration of hydronium ion [H3O+] in the reaction above for a saturated solution of o-nitrophenol? Use the ICE chart and the results from the previous two questions. You may assume that x is very small.
20. Using the results from the previous question, what is the percent ionization of a saturated solution of o-nitrophenol?
21. Using the result from problem 19, what is the pH of a saturated aqueous solution of o- nitrophenol?
22. What would happen to the equilibrium in question 17 if a strong acid, like HCl, was added? (shift to the right / shift to the left / no change)
23. What would happen to the pH if some salt of the conjugate base, Na+[C6H4NO3]-, was added to the solution in question 17? (raise / lower / no change)
24. If a buffer solution were prepared from 420 mg of o-nitrophenol [C6H5NO3] and 260 mg of its conjugate base, Na+[C6H4NO3]-, resulting in a 500 mL aqueous solution, what would the pH be?
25. What is the pH of a 0.133 M aqueous solution of NH3 given that the Ka of NH4+ is 5.68x10-10 ?
6. Which of the following acids along with their conjugate base would be best to prepare a solution buffered at 5.50? (hint: use a table of Ka values)
a) trifluoroacetic acid
b) hydrochloric acid
c) acetic acid
d) nitric acid
e) oxalic acid
H H K = 6.0 x 10-8 HO H30+ + NO2 4- NO2 0-I HStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


