Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the following balanced equation together with the data from the tables of the following groups to answer the questions CaCl2 (ac) + Na2CO3 (ac)
Use the following balanced equation together with the data from the tables of the following groups to answer the questions
CaCl2 (ac) + Na2CO3 (ac) CaCO3 (s) + 2 NaCl (ac)
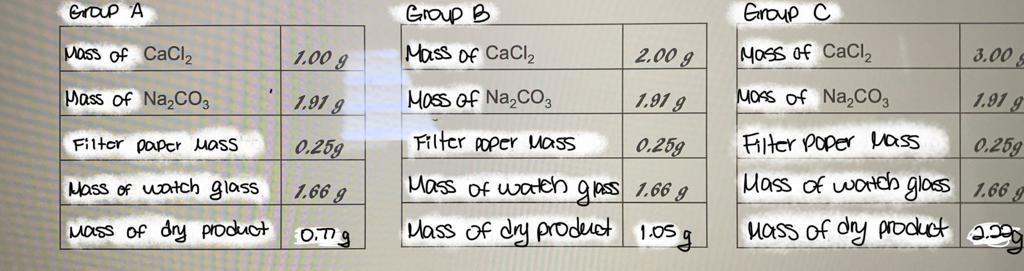
1. Determine the limiting reactant for group A, B, and C. Show your answer with calculations.
2. Calculate the theoretical yield (the maximum amount of CaCO3 that can be produced from the limiting reagent). -Group A
-B Group
-Group C
3. Determine the percentage of return. Group A:
Group B:
Group C:
Grap A Group B P Group C C Mass of CaCl2 1.00 g Mass Of CaCl2 2.00 g Moss of CaCl2 3.00 Mass of Na2CO3 1.91 g Moss of Na,C03 1.91 g 1.91 g Filter Paper Mass 0.259 MOSS of NaCO3 Filter poper Mass 0.259 Mass of watch glass 1.66 g Mass of dry product 2.999 Hass of watch glass 1.66 g Filter poper Mass 0.259 Mass of watch glass 1.66 g Mass of dry product 1.05g mass of dry product 0.779Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


