Answered step by step
Verified Expert Solution
Question
1 Approved Answer
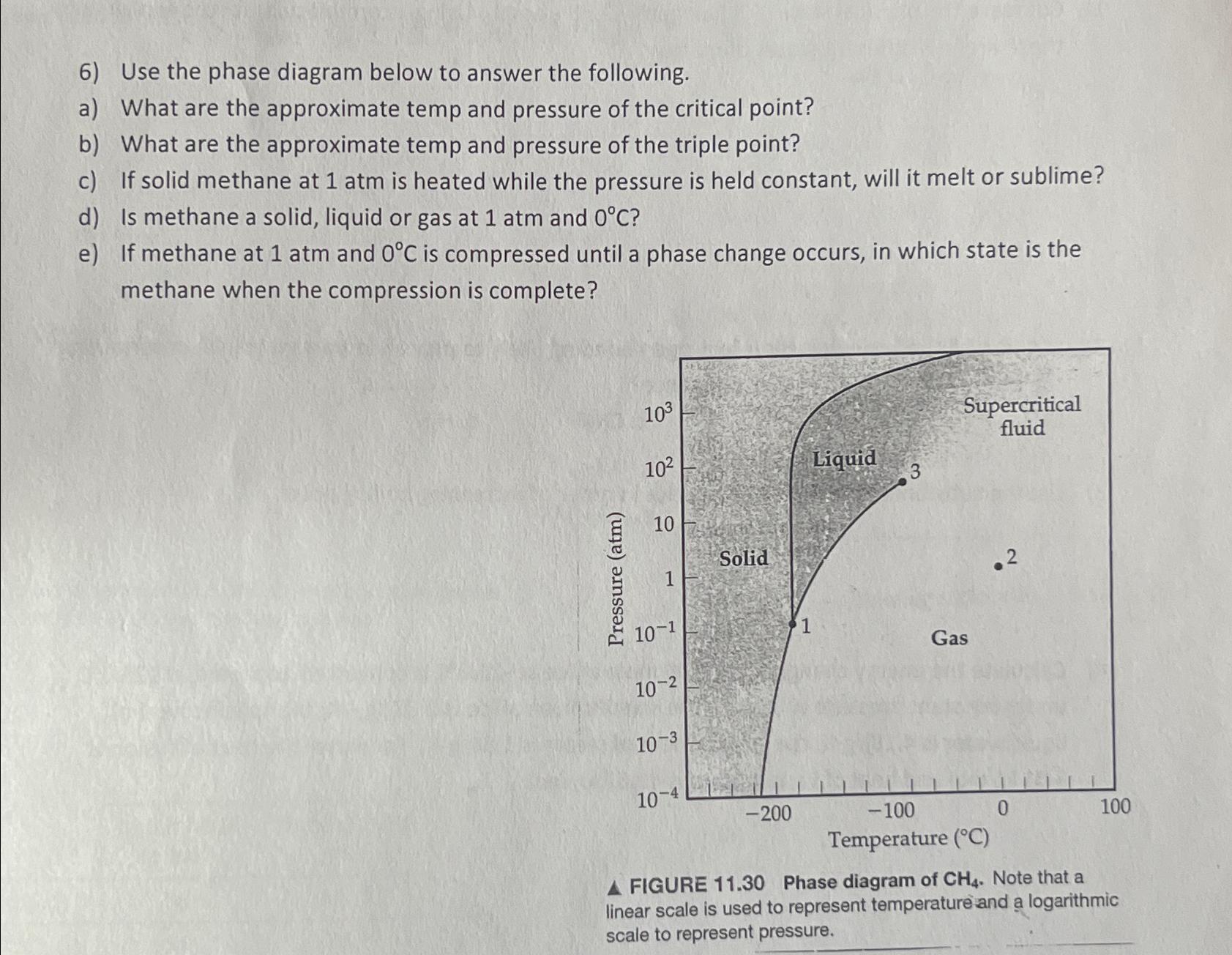
Use the phase diagram below to answer the following. a ) What are the approximate temp and pressure of the critical point? b ) What
Use the phase diagram below to answer the following.
a What are the approximate temp and pressure of the critical point?
b What are the approximate temp and pressure of the triple point?
c If solid methane at atm is heated while the pressure is held constant, will it melt or sublime?
d Is methane a solid, liquid or gas at atm and
e If methane at atm and is compressed until a phase change occurs, in which state is the methane when the compression is complete?
FIGURE Phase diagram of Note that a linear scale is used to represent temperature and a logarithmic scale to represent pressure.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


