Answered step by step
Verified Expert Solution
Question
1 Approved Answer
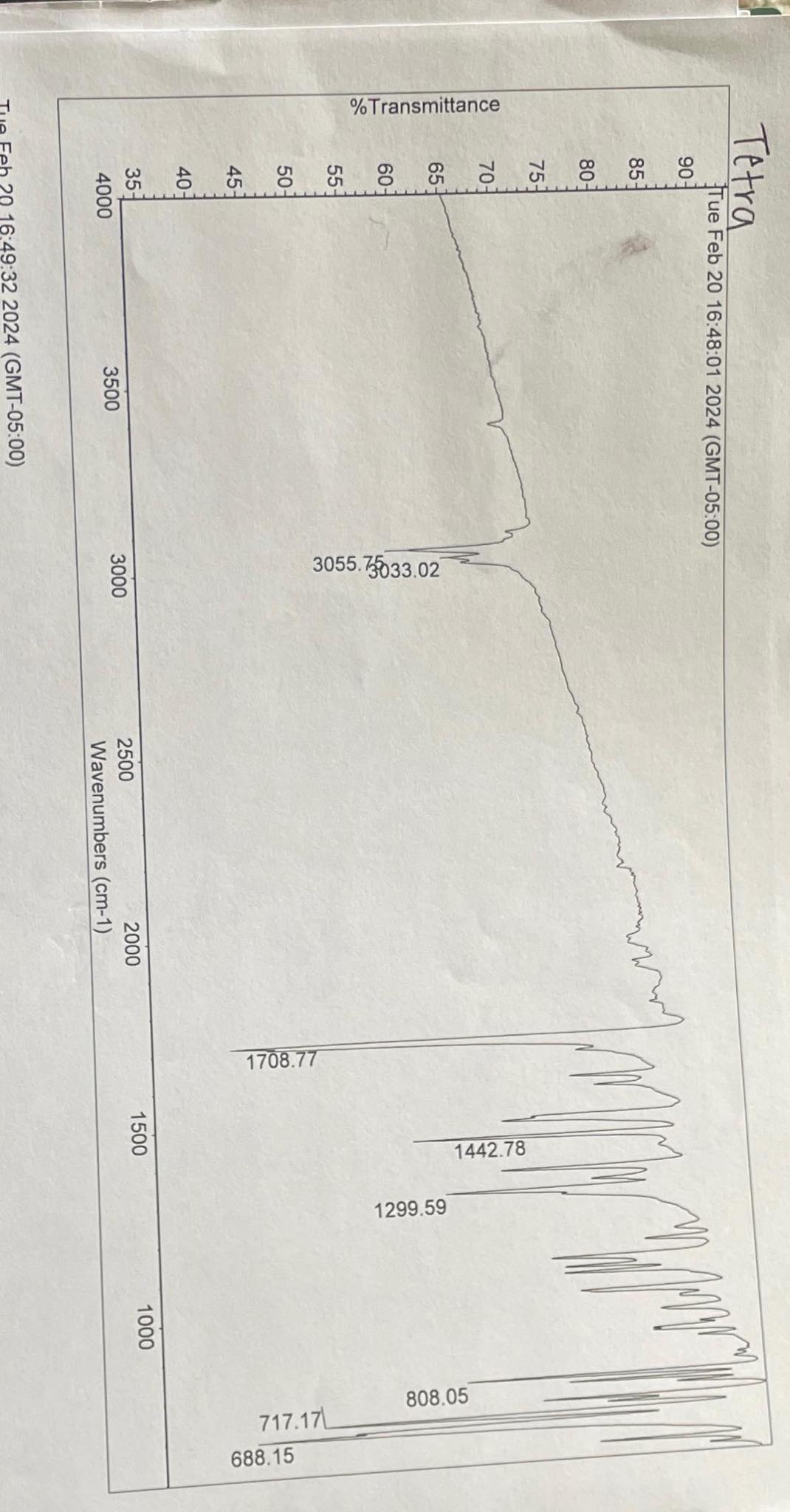
Use the technique LISTED BELOW to assign the peaks to the functional group vibrations LISTED BELOW. MOre than one peak ( label ) may appear
Use the technique LISTED BELOW to assign the peaks to the functional group vibrations LISTED BELOW. MOre than one peak label may appear for a "vibration" of a functional group.
Functional Group Vibration:
Aromatic CH stretching Vibration:
Ketone CO stretching Vibration:
Oop bending vibration on monosubtitued aromatic ring phenyl
Methyl, Methylene, and Methine
CH andCH and CH ssp CH bonds
Asymmetric bend and scissor
Symmetric bend methyl group
Gemdimethyl doublet and tertbutylw
Rocking, for chains carbons
Propyl chain
Ethyl chain
Alkenes
CH asymmetric stretch m to ssp CH bonds
CHR asymmetric stretch m to sspCH bonds
RCHCHw
RCCHw
cisRCHCHR w
transRCHCHR w
RCCHR and RCCRw
RHCCH out of plane bending oops
RCCHoops
cisRCHCHR oops
transRCHCHR oops
Rings
cyclopropane asymmetric stretch
cyclopropane symmetric stretch
Epoxides
epoxide symmetric ring stretch vs
epoxide asymmetric stretching of ring
Alkynes
CH s sharp
CC internal
CC external
Nitriles
CN stretch s sharp
Aromatic Hydrocarbons
CH asymmetric stretch
benzene substitution pattern region
monosubstituted benzene oop m to s
ortho substituted benzene oopm to s
para substituted benzene oopm to s
meta substituted benzene oopm to s
Carbonyls
Carbonyl
ketones vs
aldehydes vs
aldehyde CH w peaks
to cm for each CC attached to carbonyl s
diketones
Carboxylic Acids
OH dimer peak broad
coupled CO stretch in COOH
coupled CO stretch in COOH
OH oop
CO
Esters
Ester CO
Ester CO
methyl ester triplet
Anhydrides
CO s two bands
Ethers
OCH
methyl ether band
Alcohols
primary alcohols dilute
secondary alcohols dilute
tertiary alcohols dilute
aromatic alcohols dilute
alcohol dimers s broad
alcohols polymers s broad
secondary and tertiary alcohols
cyclic alcohols
primary alcohols
Amines
primary amines doublet secondary amines singlets broad
NH primary amine deformation
NH secondary amine deformation
aromatic amines
ammonium band
Nmethyl amines
Amides
amide carbonyl amide I band
amide II band
Chlorides
CCl stretch
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


