Question
1- Air undergoes a polytropic compression in a piston-cylinder assembly from p, = 1 bar, T = 22 C to p; = 5.5 bars.

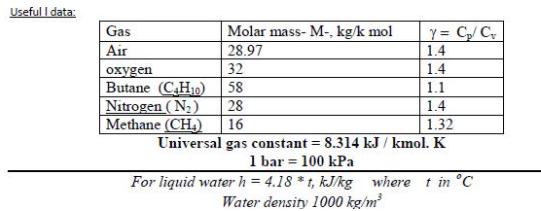
1- Air undergoes a polytropic compression in a piston-cylinder assembly from p, = 1 bar, T = 22 C to p; = 5.5 bars. Employing the ideal gas model, determine the work and heat transfer per unit mass, in kJ/kg, if n= 1.3. Useful I data: Y= C/C 1.4 Gas Molar mass- M-, kg/k mol Air 28.97 32 Butane (CH10) Nitrogen (N) oxygen 1.4 58 1.1 28 1.4 Methane (CH.) 16 1.32 Universal gas constant = 8.314 kJ / kmol. K 1 bar = 100 kPa For liquid waterh = 4.18 *t, kJkg where t in C Water density 1000 kg/m %3D
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of heat transfer
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn
7th Edition
495667706, 978-0495667704
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



