Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2- A rigid tank contains 0.5 kg of oxygen (O,) initially at 30 bar and 200 K. The gas is cooled and the pressure

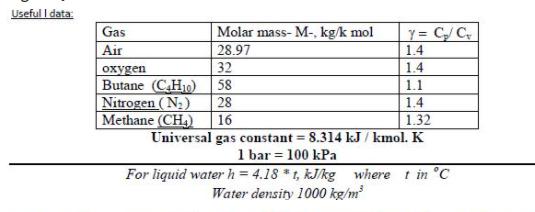
2- A rigid tank contains 0.5 kg of oxygen (O,) initially at 30 bar and 200 K. The gas is cooled and the pressure drops to 20 bar. Determine the volume of the tank, in m', and the final temperature, in K. 3 Useful I data: y = C/C 1.4 Gas Molar mass- M-, kg/k mol Air 28.97 oxygen 32 1.4 Butane (CH10) 58 1.1 1.4 1.32 Nitrogen (N;) 28 Methane (CH,) 16 Universal gas constant = 8.314 kJ / kmol. K 1 bar = 100 kPa For liquid waterh = 4.18 *t, kJ/kg where t in C Water density 1000 kg/m
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


