Answered step by step
Verified Expert Solution
Question
1 Approved Answer
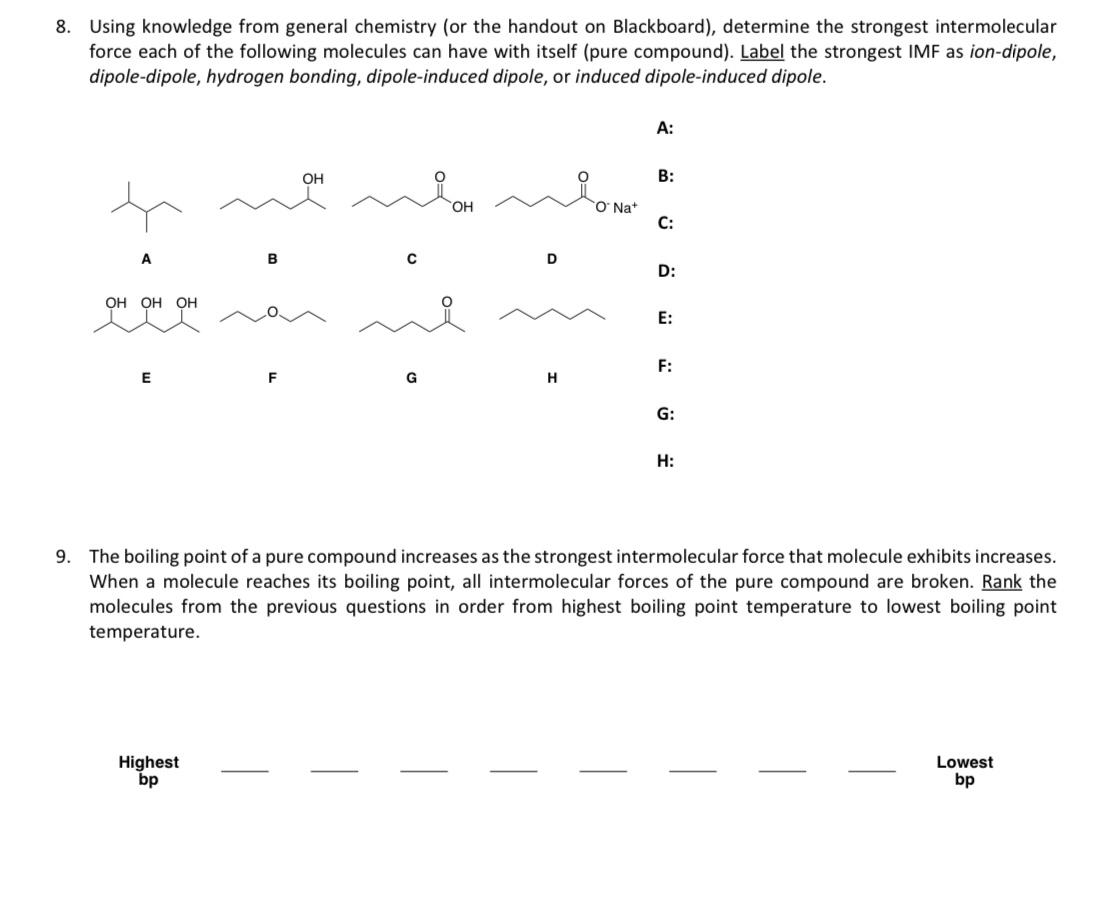
Using knowledge from general chemistry ( or the handout on Blackboard ) , determine the strongest intermolecular force each of the following molecules can have
Using knowledge from general chemistry or the handout on Blackboard determine the strongest intermolecular force each of the following molecules can have with itself pure compound Label the strongest IMF as iondipole, dipoledipole, hydrogen bonding, dipoleinduced dipole, or induced dipoleinduced dipole.
A:
:
H:
The boiling point of a pure compound increases as the strongest intermolecular force that molecule exhibits increases. When a molecule reaches its boiling point, all intermolecular forces of the pure compound are broken. Rank the molecules from the previous questions in order from highest boiling point temperature to lowest boiling point temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


