Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = x A Preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions

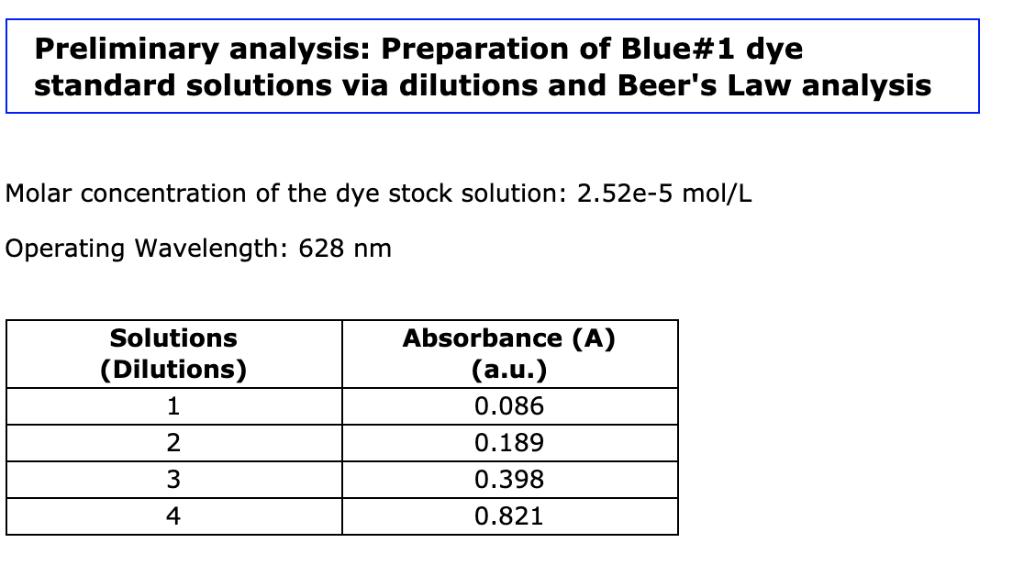

Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = x A Preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions and Beer's Law analysis Molar concentration of the dye stock solution: 2.52e-5 mol/L Operating Wavelength: 628 nm Solutions (Dilutions) 1 2 3 4 Absorbance (A) (a.u.) 0.086 0.189 0.398 0.821 Solution 1: 1.00 mL of Blue#1 Stock Solution and 19.00 mL of distilled water Solution 2: 2.00 mL of Blue#1 Stock Solution and 18.00 mL of distilled water Solution 3: 4.00 mL of Blue#1 Stock Solution and 16.00 mL of distilled water Solution 4: 8.00 mL of Blue#1 Stock Solution and 12.00 mL of distilled water From the preliminary analysis: Preparation of Blue#1 dye standard solutions via dilutions and Beer's Law analysis Using linear regression determine the absorbance/concentration relationship for the dye. [dye] = A
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Answer Concentration of initial stock solution 25210 5 molL or M For dilution of Stock solut...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


