Answered step by step
Verified Expert Solution
Question
1 Approved Answer
USING MATLAB The saturation concentration of dissolved oxygen in freshwater can be calculated with the equation ln o_sf = -139.34411 + 1.575701 times 10^5/T_a -
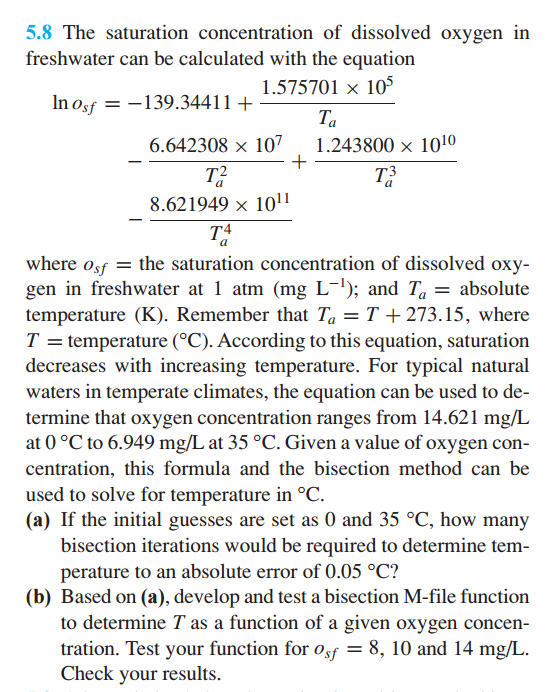
USING MATLAB
The saturation concentration of dissolved oxygen in freshwater can be calculated with the equation ln o_sf = -139.34411 + 1.575701 times 10^5/T_a - 6.642308 times 10^7/T_a^2 + 1.243800 times 10^10/T_a^3 - 8.621949 times 10^11/T_a^4 where osf = the saturation concentration of dissolved oxygen in freshwater at 1 atm (mg L^-1); and T_a = absolute temperature (K). Remember that T_a = T + 273.15, where T temperature (degree C). According to this equation, saturation decreases with increasing temperature. For typical natural waters in temperate climates, the equation can be used to determine that oxygen concentration ranges from 14.621 mg/L at 0 degree C to 6.949 mg/L at 35 degree C. Given a value of oxygen concentration, this formula and the bisection method can be used to solve for temperature in degree C. If the initial guesses are set as 0 and 35 degree C, how many bisection iterations would be required to determine temperature to an absolute error of 0.05 degree C? Based on (a), develop and test a bisection M-file function to determine T as a function of a given oxygen concentration. Test your function for o_sf = 8, 10 and 14 mg/L. Check your resultsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


