Answered step by step
Verified Expert Solution
Question
1 Approved Answer
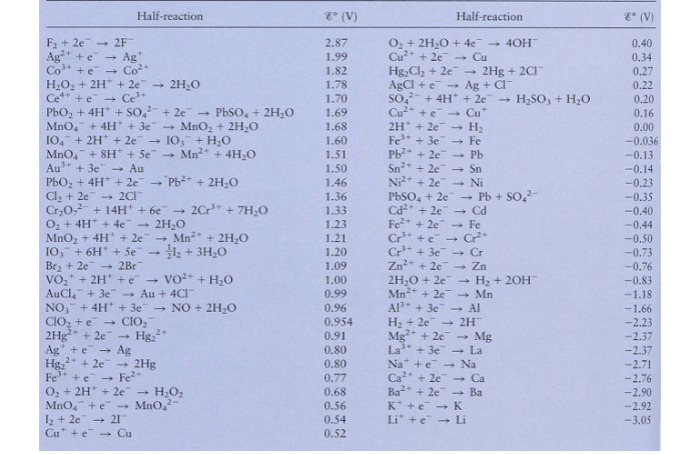
Using Table 1 8 . 1 from your book and / or the list of standard reduction potentials on the next page, calculate the standard
Using Table from your book andor the list of standard reduction potentials on the next
page, calculate the standard cell potential for each of the following types of batteries and give
the corresponding overall reaction:
A normal AA battery is typically a type of alkaline battery, also known as an alkaline
manganese battery. It contains a zinc anode negative terminal a manganese dioxide
cathode positive terminal and an alkaline electrolyte solution. The electrochemical
reactions that take place within the battery involve:
the anode: Zns Znaqe
the cathode: MnOsHaqe MnaqHOl
A normal car battery is typically a leadacid battery, which consists of lead dioxide
PbO as the positive electrode cathode sponge lead Pb as the negative electrode
anode and a sulfuric acid HSO electrolyte solution. The electrochemical reactions
that occur within the battery involve:
the anode: Pbs Pbaqe
the cathode: PbOsHaq SOaqe PbSOsHOl
In a lithiumion battery, the electrochemical reactions involve lithium ions Li moving
between a graphitebased anode and a metal oxide cathode, such as cobaltIII oxide.
Here are the simplified halfreactions that occur during the discharge of a lithiumion
battery:
the anode: Lis Liaq e
the cathode: Coaq e Coaq
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


