Question: Using the curved arrow to guide your reasoning, show the products of the following dissociations. Include formal charges and unshared electron pairs. Check your
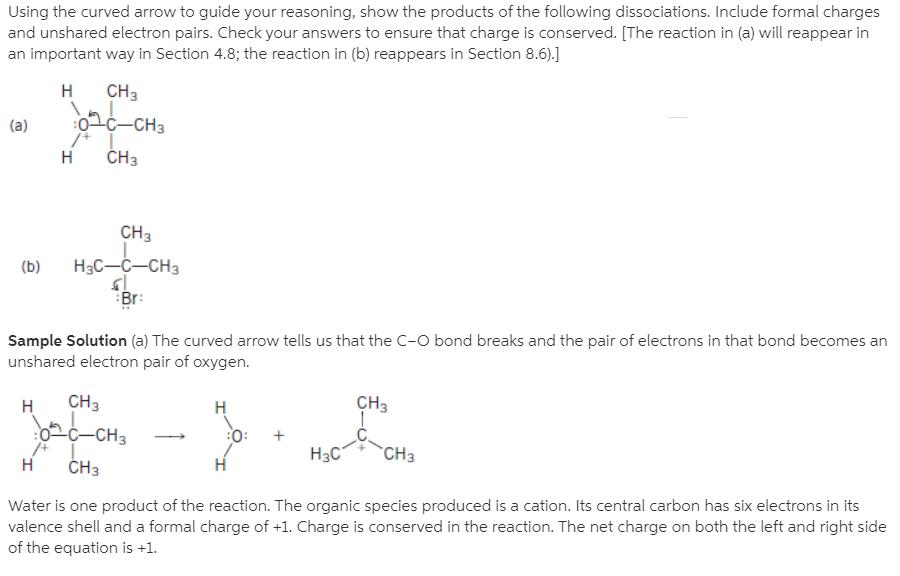
Using the curved arrow to guide your reasoning, show the products of the following dissociations. Include formal charges and unshared electron pairs. Check your answers to ensure that charge is conserved. [The reaction in (a) will reappear in an important way in Section 4.8; the reaction in (b) reappears in Section 8.6).] CH3 on0-CH3 (a) H3 CH (b) Br: Sample Solution (a) The curved arrow tells us that the C-O bond breaks and the pair of electrons in that bond becomes an unshared electron pair of oxygen. CH3 0C-CH3 CH :O: "C H3 Water is one product of the reaction. The organic species produced is a cation. Its central carbon has six electrons in its valence shell and a formal charge of +1. Charge is conserved in the reaction. The net charge on both the left and right side of the equation is +1.
Step by Step Solution
3.29 Rating (152 Votes )
There are 3 Steps involved in it
To solve part b follow these steps Step 1 Identify the Curved Arrow Notation The curved arrow indica... View full answer

Get step-by-step solutions from verified subject matter experts


