Answered step by step
Verified Expert Solution
Question
1 Approved Answer
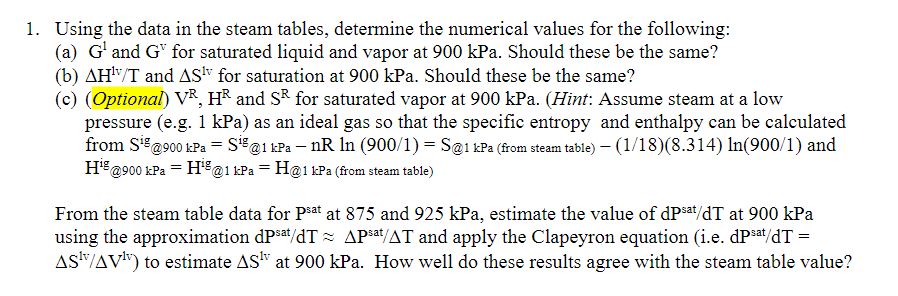
Using the data in the steam tables, determine the numerical values for the following: ( a ) G 1 and G v for saturated liquid
Using the data in the steam tables, determine the numerical values for the following:
a and for saturated liquid and vapor at kPa. Should these be the same?
b and for saturation at kPa. Should these be the same?
cOptional and for saturated vapor at kPa. Hint: Assume steam at a low
pressure egkPa as an ideal gas so that the specific entropy and enthalpy can be calculated
from @kPa@kPafrom steam table and
from steam table
From the steam table data for at and kPa, estimate the value of at kPa
using the approximation ~~ and apply the Clapeyron equation ie
to estimate at kPa. How well do these results agree with the steam table value?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


