Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Using the data of Table I, calculate the adiabatic constant-pressure flame temperature for boiler with a fuel blend as equimolar mixture of propane (C3H8) and
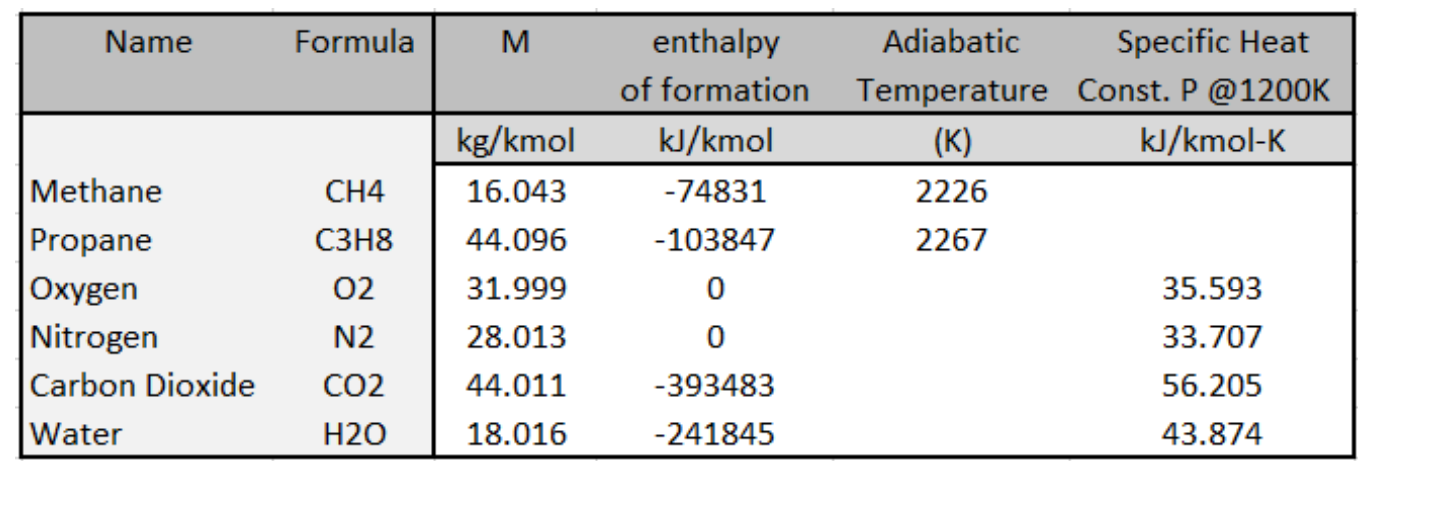 Using the data of Table I, calculate the adiabatic constant-pressure flame temperature for boiler with a fuel blend as equimolar mixture of propane (C3H8) and natural gas (CH4) and equivalence ratio of 0.8. Assume complete combustion of CO2 and H2O, neglect dissociation. Also, assume the heat capacity of combustion products s constant evaluated at 1200K. The boiler operates at 1 atm., and both air and fuel enter at 298K. \begin{tabular}{|lc|cccc|} \hline \multicolumn{1}{|c|}{ Name } & Formula & M & enthalpyofformation & AdiabaticTemperature & SpecificHeatConst.P@1200K \\ \hline \multirow{3}{*}{ Methane } & & kg/kmol & kJ/kmol & (K) & kJ/kmolK \\ \cline { 3 - 6 } Propane & CH4 & 16.043 & -74831 & 2226 & \\ Oxygen & C3H8 & 44.096 & -103847 & 2267 & \\ Nitrogen & O2 & 31.999 & 0 & & 35.593 \\ Carbon Dioxide & N2 & 28.013 & 0 & & 33.707 \\ Water & CO2 & 44.011 & -393483 & & 56.205 \\ \hline \end{tabular}
Using the data of Table I, calculate the adiabatic constant-pressure flame temperature for boiler with a fuel blend as equimolar mixture of propane (C3H8) and natural gas (CH4) and equivalence ratio of 0.8. Assume complete combustion of CO2 and H2O, neglect dissociation. Also, assume the heat capacity of combustion products s constant evaluated at 1200K. The boiler operates at 1 atm., and both air and fuel enter at 298K. \begin{tabular}{|lc|cccc|} \hline \multicolumn{1}{|c|}{ Name } & Formula & M & enthalpyofformation & AdiabaticTemperature & SpecificHeatConst.P@1200K \\ \hline \multirow{3}{*}{ Methane } & & kg/kmol & kJ/kmol & (K) & kJ/kmolK \\ \cline { 3 - 6 } Propane & CH4 & 16.043 & -74831 & 2226 & \\ Oxygen & C3H8 & 44.096 & -103847 & 2267 & \\ Nitrogen & O2 & 31.999 & 0 & & 35.593 \\ Carbon Dioxide & N2 & 28.013 & 0 & & 33.707 \\ Water & CO2 & 44.011 & -393483 & & 56.205 \\ \hline \end{tabular} Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


