Question
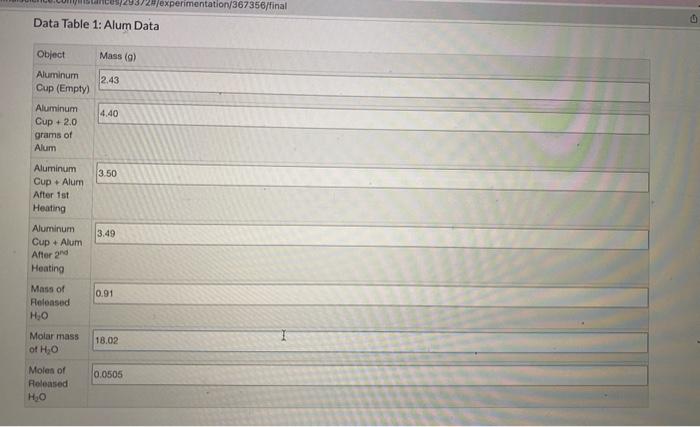
USING THE DATA TABLE PLEASE HELP ANSWER THESE PARTS OF THE STUDY GUIDE. A. Calculate the moles of anhydrous (dry) KAI(SO4)2 that were present in
USING THE DATA TABLE PLEASE HELP ANSWER THESE PARTS OF THE STUDY GUIDE.
A. Calculate the moles of anhydrous (dry) KAI(SO4)2 that were present in the sample. Show all work including units.
B.Calculate the ratio of moles of H2O to moles of anhydrous KAI(SO4)2. Show all work including units.
Note: Report the ratio to the closest whole number.
C. Write the empirical formula for the hydrated KAI(SO4)2, based on your experimental results and answer to Question 2. Show all work including units.
Hint: if the ratio of moles of H2O to moles of anhydrous KAI(SO4)2 was 4, then the empirical formula would be: KAI(SO4)24H20.
D. Describe any visual differences between the hydrated sample and the dried, anhydrous form.
E. How would the following errors affect the empirical formula for the compound? That is, will these errors cause the calculated number of moles of water in the hydrate to be artificially high or low?
- The student ran out of time and did not do the second heating. Explain how this erfor will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low?
- The student recorded the mass of the cup + sample incorrectly and started with 2.20 g of hydrated compound but used 2.00 g in the calculations. Explain how this error will affect the calculation for the number of moles of water in the hydrate? Will the final answer be artificially high or low? How do you know?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


