Answered step by step
Verified Expert Solution
Question
1 Approved Answer
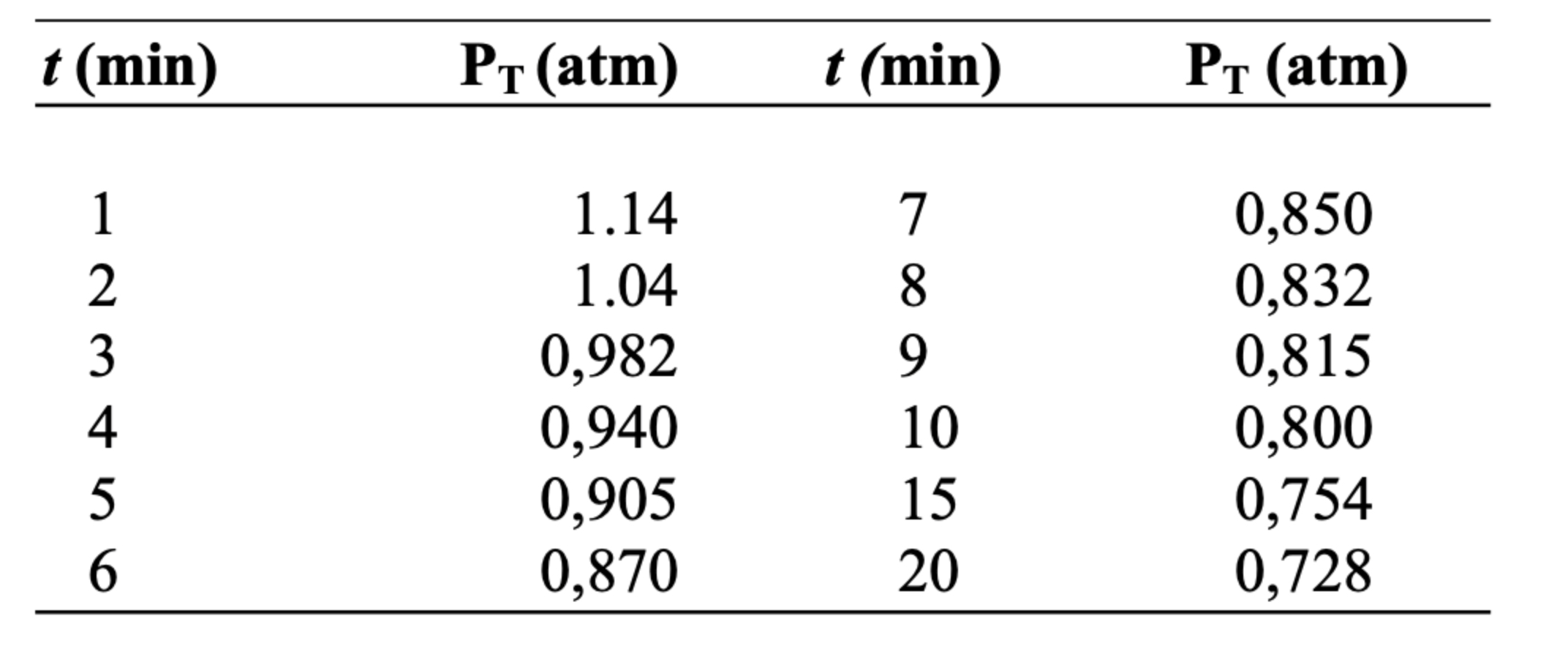
Using the differentiual method solve the following: A small reaction pump, equipped with a sensitive device for measuring pressure, is evacuated and then filled with
Using the differentiual method solve the following:
A small reaction pump, equipped with a sensitive device for measuring
pressure, is evacuated and then filled with pure reactant A at a pressure of atm. The operation is carried out at C a temperature low enough so that the reaction does not occur in an appreciable extension.
The temperature is raised as quickly as possible to C by immersing the pump in boiling water, from which the data given in the table is obtained. The stoichiometric equation for the reaction is A B and after the bomb remains in the bath for a long time it performs an analysis to find out the amount of reactant A and it is found that this component has ran out. Derive the kinetic equation that fits these data, expressing the units in mole, liter and minute.
Note: Pt is the total preassure
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


