Answered step by step
Verified Expert Solution
Question
1 Approved Answer
using the info provided can i plz have help with answer all parts of these qs neatly and clearly (part B and C) Test 1:Ume
using the info provided can i plz have help with answer all parts of these qs neatly and clearly (part B and C)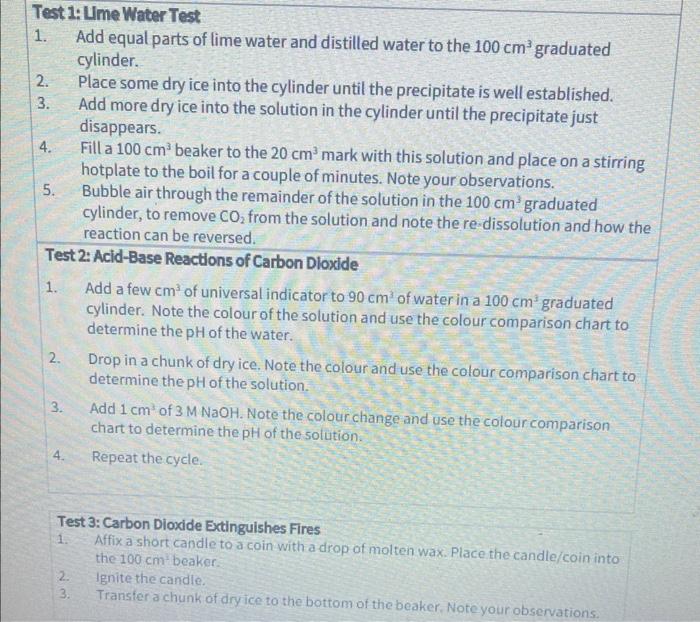
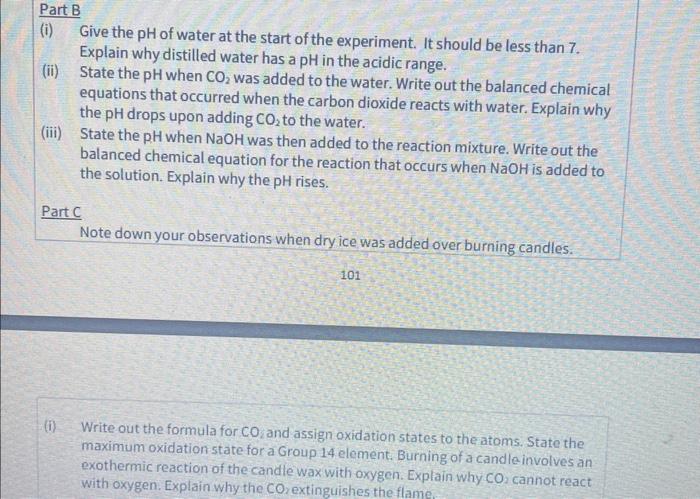
Test 1:Ume Water Test 1. Add equal parts of lime water and distilled water to the 100cm3 graduated cylinder. 2. Place some dry ice into the cylinder until the precipitate is well established. 3. Add more dry ice into the solution in the cylinder until the precipitate just disappears. 4. Fill a 100cm3 beaker to the 20cm3 mark with this solution and place on a stirring hotplate to the boil for a couple of minutes. Note your observations. 5. Bubble air through the remainder of the solution in the 100cm3 graduated cylinder, to remove CO2 from the solution and note the re.dissolution and how the reaction can be reversed. Test 2: Acid-Base Reactions of Carbon Dloxide 1. Add a few cm3 of universal indicator to 90cm3 of water in a 100cm3 graduated cylinder. Note the colour of the solution and use the colour comparison chart to determine the pH of the water. 2. Drop in a chunk of dry ice. Note the colour and use the colour comparison chart to determine the pH of the solution. 3. Add 1cm3 of 3MNaOH. Note the colour change and use the colour comparison chart to determine the pH of the solution. 4. Repeat the cycle. Test 3: Carbon Dioxide Extinguishes Fires 1. Affix a short candle to a coin with a drop of molten wax. Place the candle/coin into the 100cmbeaker. 2. Ignite the candle. 3. Transfer a chunk of dry ice to the bottom of the beaker. Note your observations. (i) Give the pH of water at the start of the experiment. It should be less than 7 . Explain why distilled water has a pH in the acidic range. (ii) State the pH when CO2 was added to the water. Write out the balanced chemical equations that occurred when the carbon dioxide reacts with water. Explain why the pH drops upon adding CO2 to the water. (iii) State the pH when NaOH was then added to the reaction mixture. Write out the balanced chemical equation for the reaction that occurs when NaOH is added to the solution. Explain why the pH rises. Part C Note down your observations when dry ice was added over burning candles. 101 maximum oxidation state for a Group 14 element. Burning of a candle involves an exothermic reaction of the candle wax with oxygen. Explain why CO2 cannot react with oxygen. Explain why the CO extinguishes the flame 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


