Question
Using the single-step global mechanism for combustion of a hydrocarbon with air, compare the mass rates of fuel carbon conversion to CO, for 0 =1,
Using the single-step global mechanism for combustion of a hydrocarbon with air,
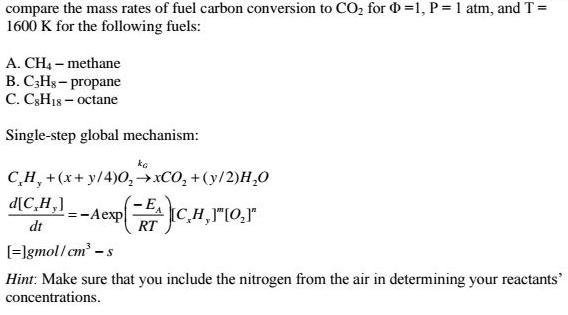
compare the mass rates of fuel carbon conversion to CO, for 0 =1, P= 1 atm, and T = 1600 K for the following fuels: A. CH4 - methane B. C;Hg- propane C. C3H18 octane Single-step global mechanism: C,H, +(x+ y/4)0,XCO, + (y/2)H,0 d[C,H,] - E, :-Aexp CH,"[0,r" dt RT [=]gmol/cm - Hint: Make sure that you include the nitrogen from the air in determining your reactants' concentrations.
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Robert Thornton Morrison, Robert Neilson Boyd
6th Edition
8120307208, 978-8120307209
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



