Using the tables below from Perry's chemical engineering handbook determine the following:
- Viscosity of diethyl ether (in Pascal-second) at 77 degrees Celsius
- Viscosity (Pascal-second) of cyclohexene at 50 degrees Celsius.
- The density (in kg per cubic meter) of cyclohexene at 50 degrees Celsius
- The kinematic viscosity (in sq. m per second) of cyclohexene at 50 degrees Celsius.]]
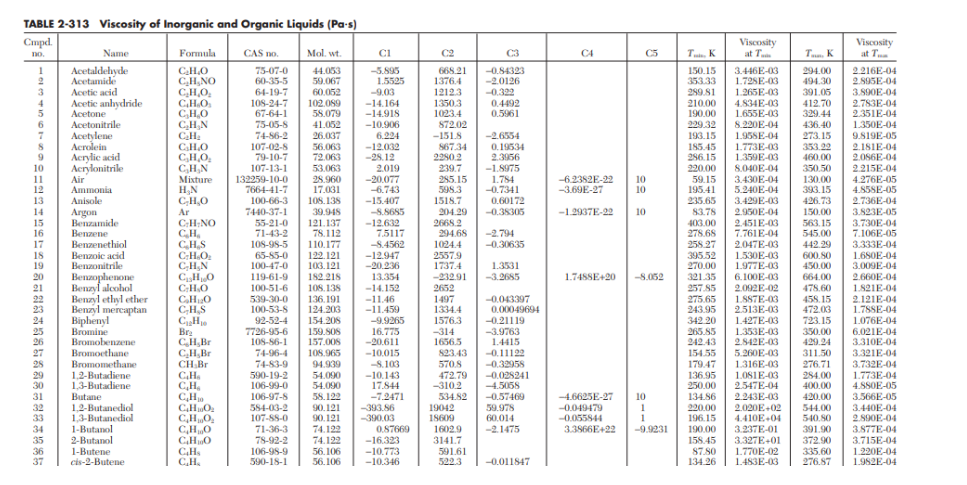
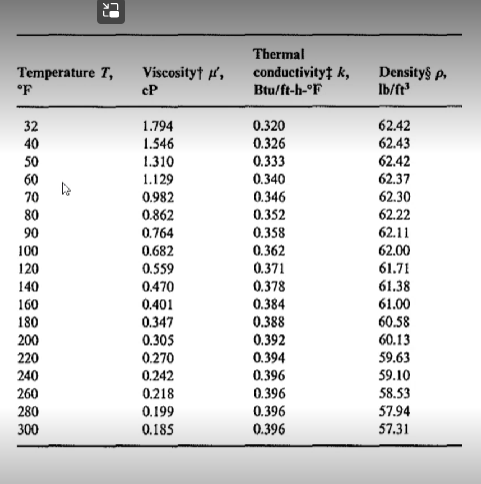
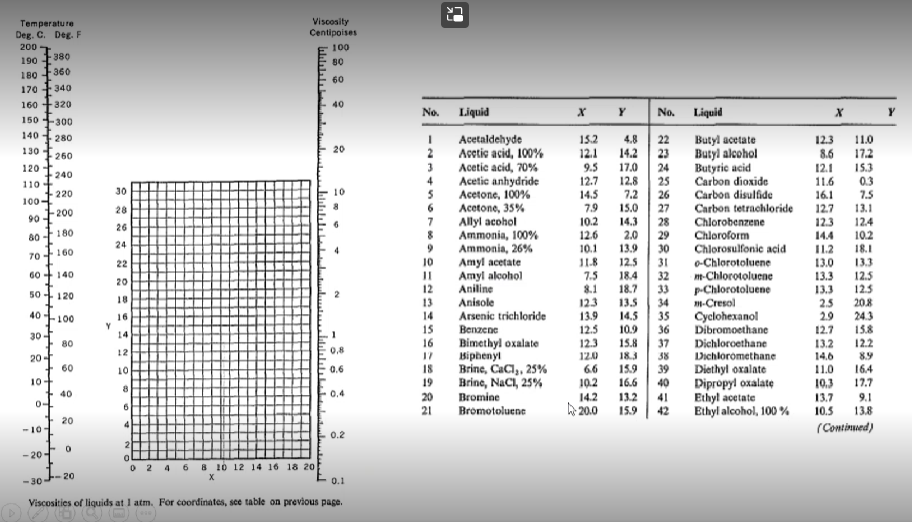
C2 C3 CA CS T... K -0.84323 -2.0126 -0.322 0.4492 0.5961 0.19534 2.3956 -1.8975 1.784 -0.7341 0.60172 -0.38305 -6.2352E-22 -3.69E-27 10 10 -1.2937E-22 10 TABLE 2-313 Viscosity of Inorganic and Organic Liquids (Pa-s) Cmpd. TO Name Formula CAS no Mol. wt. 1 Acetaldehyde CHO 75-07-0 44.053 -5.895 2 Acetamid CH.NO 60-35-5 59.067 1.5525 3 Acetic acid CHO 64-19-7 60,052 -9.03 4 Acetic anhydride CHO 10S-24-7 102.099 - 14.164 5 Acetone CHO 67-64-1 58.079 - 14.915 6 Acetonitrile CHN 75-05-8 41.052 -10.906 7 Acetylene CH 74-86-2 26.037 6.024 S Acrolein CHO 107-02-8 56.063 - 12.032 9 Acrylic acid CHO 79-10-7 72.063 -28.12 10 Acrylonitrile CHN 107-13-1 53.063 2.019 11 Air Mixture 132259-10-0 25.960 -20.077 12 Ammonia HN 766-1-41-7 17.031 -6.743 13 Anisole CHO 100-66-3 105. 135 -15.407 14 Argon Ar 7440-37-1 39.945 -8.5685 15 Benzamide C.H.NO 55-21-0 121.137 -12.632 16 Benzene CH 71-43-2 78.112 7.5117 17 Benzenethiol C.HS 109-98-5 110.177 -8.4562 18 Benzoic acid C.H.O 65-85-0 122.121 -12.947 19 Benzonitrile 100-47-0 103.121 -20.236 20 Benzophenone CHO 119-61-9 152.215 13.351 21 Benzyl alcohol C.H.O 100-51-6 105. 138 -14.152 92 Benzyl ethyl ether CHO 539-30-0 136.191 -11.46 23 Benzyl mercaptan CHS 100-53-8 124.203 -11.459 24 Biphenyl CH 92-52-4 154.209 -9.9265 25 Bromine Bry 7726-95-6 159.SOS 16.7775 26 Bromobenzene CH,BE 105-S6-1 157.00 -20.611 27 Bromoethane CH, Br 74-96-4 108.965 -10.015 28 Bromomethane CH.BI 74-83-9 94.939 -8.103 29 1.2-Butadiene CH 590-19-2 54.090 -10.143 30 13-Butadiene CH, 106-99-0 54.090 17.844 31 Butane C.H. 106-97-8 58. 122 -7.2471 32 1.2-Butanediol CHO 584-03-2 90.121 -393.86 1,3-Butanediol CHO, 107-SS-O 90.121 -390.03 1-Butanol CHO 71-36-3 74.122 0.87669 2-Butanol CHO 78-92-2 74.122 -16.323 1-Butene CH 106-95-9 56.106 -10.773 37 cis-2-Butene CH 590-18-1 56.106 -10.346 -2.794 -0.30635 668.21 1376.4 12123 13503 1023.4 872.02 -151.8 56734 2290.2 239.7 295.15 595.3 1518.7 204.29 2665.2 294.68 1024.4 2557.9 1737.4 -232.91 2652 1497 1334.4 1576.3 -314 1656.5 823.43 570.8 472.79 -3102 534.82 19042 18609 1602.9 31417 591.61 522.3 Viscosity at ... 3.446E-03 1.725E-03 1 265F-03 4.834E-03 1.655E-03 8.220E-01 1.958E-04 1.773E-03 1.359E-03 8.040E-04 3.430E-04 5.240E-04 3.429E-03 2.950E-04 2.451E-03 7.761E-04 2.047E-03 1.530E-03 1.977E-03 6.100E-03 2.092E-02 1.887E-03 2.513E-03 1.427E-03 1.353E-03 2.842E-03 5.260E-03 1.316E-03 1.OSIE-03 2.547E-04 2.243E-03 2.020E+02 4.410E-04 3.237E-01 3.327E+01 1.770E-02 1.483E-03 . 150.15 35333 299 S1 210.00 190.00 299.32 193.15 185.45 256.15 220.00 59.15 195.41 235.65 83.78 103.00 278.68 258 27 395.52 270.00 321.35 257.85 275.65 243.95 342 20 265.85 242.43 154.55 179.47 136.95 250.00 134.56 920,00 196.15 190.00 158.45 87.80 134.26 CHN Viscosity at T 2.216E-04 2.895E-04 3.990E-04 2.783E-04 2.351E-04 1.350E-04 9.819E-05 2.181E-04 2.056E-04 2215E-04 4.276E-05 4.85SE-05 2.736E-04 3.923E-05 3.730E-04 7.106E-05 3.333E-04 1.680E-04 3.009E-04 2.660E-04 1.921E-04 2.121E-04 1.78SE-04 1.076E-04 6.021E-04 3.310E-04 3.321E-04 3.732E-04 1.773E-04 4.SSOE-05 3.566E-05 3.440E-04 2.890E-04 3.877E-04 3.715E-04 1.220E-04 1.952E-04 994.00 494.30 391.05 412.70 329.44 436.40 273.15 353.22 460.00 350.50 130.00 393.15 426.73 150.00 563.15 545.00 442.29 600.50 450.00 664.00 478.60 455.15 472.03 723.15 350.00 429.24 311.50 276.71 284.00 400.00 420.00 544.00 540.50 391.90 372.90 335.60 276.87 1.3531 -3.2685 1.74SSE 20 - 8.052 -0.013397 0.00049694 -0.21119 -3.9763 1.4415 -0.11122 -0.32955 -0.028241 -4.5058 -0.57469 59.978 60.014 -2.1475 -4.6625E-27 -0.049479 -0.055844 3.3966E+22 10 1 1 1 -9.9231 9882 -0.011847 Temperature T, OF Viscosityti, cP Thermal conductivity k, Btu/ft-b-or Densitys Ib/ft? 32 40 50 60 70 80 90 100 120 140 160 180 200 220 240 260 280 300 1.794 1.546 1.310 1.129 0.982 0.862 0.764 0.682 0.559 0.470 0.401 0.347 0.305 0.270 0.242 0.218 0.199 0.185 0.320 0.326 0.333 0.340 0.346 0.352 0.358 0.362 0.371 0.378 0.384 0.388 0.392 0.394 0.396 0.396 0.396 0.396 62.42 62.43 62.42 62.37 62.30 62.22 62.11 62.00 61.71 61.38 61.00 60.58 60.13 59.63 59.10 58.53 57.94 57.31 22 Temperature Deg. C. Deg. F 200 190 180 -360 170 - 340 160 +320 Viscosity Centipoises 100 80 60 -380 40 X Y Y 150-300 280 140 130 20 260 120 110 152 12.1 9.5 12.7 14.5 240 220 30 100- 90 200 79 28 26 80 180 24 4.8 14.2 17.0 12.8 7.2 15.0 14.3 2.0 13.9 12.5 18.4 18.7 13.5 14.5 10.9 70 160 No. Liquid Acetaldehyde Acetic acid, 100% Acetic acid, 70% 4 Acetic anhydride 5 Acetone, 100% Acetone, 35% 7 Allyl acohol 8 Ammonia, 100% 9 Ammonia, 26% Amyl acetate 11 Amyl alcohol 12 Aniline 13 Anisole 14 Arsenic trichloride 15 | Benzema 16 Bimethyl oxalate 17 Biphenyi 18 Brinc, CaCl,. 25% 19 Brinc, NaCl, 25% 20 Bromine 21 Bromotoluenc 22 No. Liquid 22 Butyl acetate 23 Butyl alcohol 24 Butyric acid 25 Carbon dioxide 26 Carbon disulfide 27 Carbon tetrachloride 28 Chlorobenzene 29 Chloroform 30 Chlorosulfonic acid 31 o-Chlorotoluene 32 m-Chlorotoluene 33 p-Chlorotoluene 34 1H-Cresol 35 Cyclohexanol 36 Dibromoethane 37 Dichloroethane 38 Dichloromethane 39 Diethyl oxalate 40 Dipropyl oxalate 41 Ethyl acetate 42 Ethyl alcohol, 100% 60+ 140 20 12.3 11.0 8.6 17.2 12.1 15.3 11.6 03 16.1 7.5 12.7 13.1 12.3 12.4 14.4 102 IL2 18.1 13.0 13.3 13.3 12.5 13.3 125 2.5 20.8 29 243 12.7 15.8 13.2 122 14,6 8.9 11.0 16.4 10.3 17.7 13.7 9.1 13.8 (Continued) 50 120 10.2 12.6 10.1 11.8 7.5 8.1 123 13.9 12.5 123 120 6.6 10.2 14.2 h 20.0 18 40-100 16 Y 14 15.8 12 0,8 30-1 80 20-1 60 10+ 40 0+ 10 0.6 183 15.9 16.6 132 15.9 8 0.4 & 6 10.5 20 4 -101 0.2 0 -20-1 2 4 6 8 10 12 14 16 18 20 -30 20 0.1 Viscosities of liquids at 1 atm. For coordinates, see table on previous page









