Question: Using the ternary diagram given below for methane (C1), normal-butane(n-C4), and normal-decane (n-C10) at 160F and 2000 psia, answer the following questions: a. Clearly identify
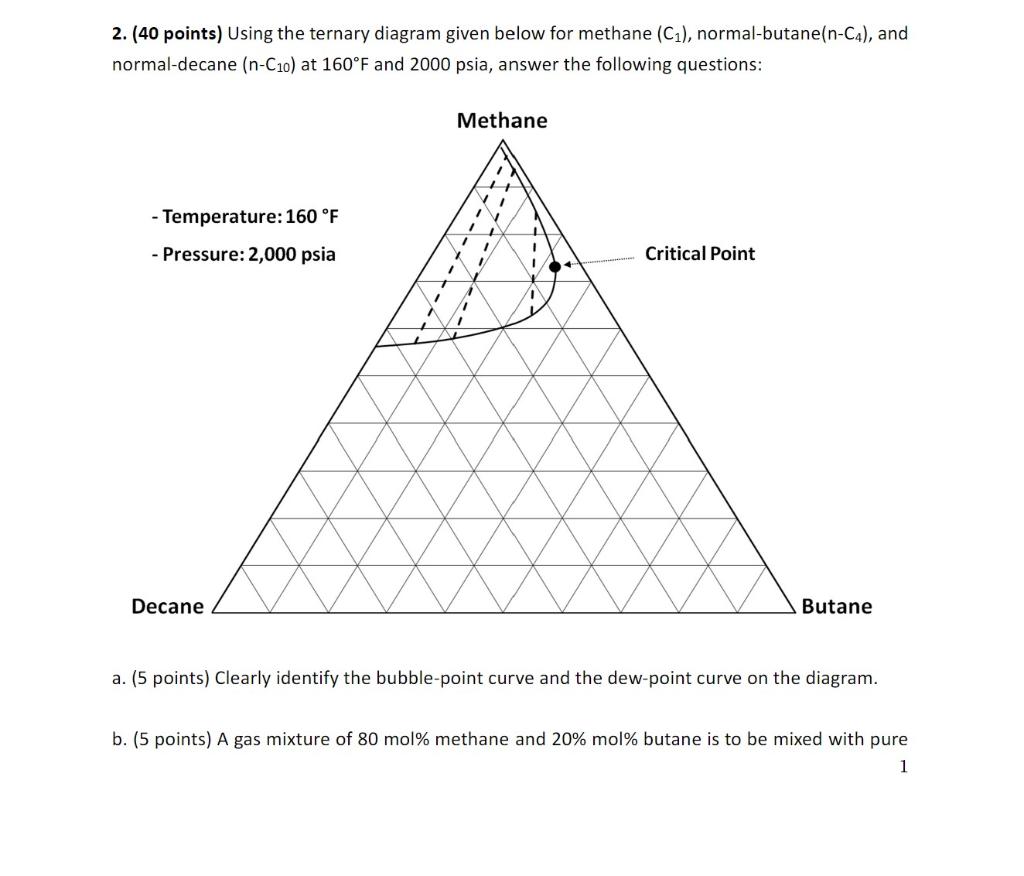

Using the ternary diagram given below for methane (C1), normal-butane(n-C4), and normal-decane (n-C10) at 160F and 2000 psia, answer the following questions:
a. Clearly identify the bubble-point curve and the dew-point curve on the diagram. b. A gas mixture of 80 mol% methane and 20% mol% butane is to be mixed with pure decane (100% decane). Will all possible mixtures of two fluids (the gas mixture and pure decane) be single phase at 160F and 2,000 psia? In other words, is the gas mixture (first-contact) miscible with pure decane at 160F and 2,000 psia? Explain why or why not, on the basis of the ternary diagram given.
c. (10 points) Consider Mixture A and Mixture B at 160F and 2,000 psia: Mixture A : 80 mol% methane, 3 mol% butane, and 17 mol% decane Mixture B : 30 mol% methane, 38 mol% butane, and 32 mol% decane Mixture C is created by mixing 80 mol% Mixture A and 20 mol% Mixture B. Calculate the composition for Mixture C. Confirm that Mixture C is in the two-phase region based on the ternary diagram given.
d. Mixture C forms two phases at 160F and 2,000 psia. Using the ternary diagram given, what is the composition of the equilibrium liquid phase? What is the composition of the equilibrium gas phase?
e. (10 points) For Mixture C, calculate the mole fraction of the liquid phase. The easiest way to answer this question may be to apply the lever rule after measuring with a ruler the tie line identified in part d.
Thank you!!
2. (40 points) Using the ternary diagram given below for methane (C1), normal-butane (nC4), and normal-decane (nC10) at 160F and 2000 psia, answer the following questions: le a. (5 points) Clearly identify the bubble-point curve and the dew-point curve on the diagram. b. (5 points) A gas mixture of 80 mol\% methane and 20% mol\% butane is to be mixed with pure 1 decane ( 100% decane). Will all possible mixtures of two fluids (the gas mixture and pure decane) be single phase at 160F and 2,000 psia? In other words, is the gas mixture (firstcontact) miscible with pure decane at 160F and 2,000 psia? Explain why or why not, on the basis of the ternary diagram given. c. (10 points) Consider Mixture A and Mixture B at 160F and 2,000 psia: Mixture A : 80 mol\% methane, 3 mol\% butane, and 17 mol\% decane Mixture B : 30 mol\% methane, 38 mol\% butane, and 32 mol\% decane Mixture C is created by mixing 80 mol\% Mixture A and 20 mol\% Mixture B. Calculate the composition for Mixture C. Confirm that Mixture C is in the two-phase region based on the ternary diagram given. d. (10 points) Mixture C forms two phases at 160F and 2,000 psia. Using the ternary diagram given, what is the composition of the equilibrium liquid phase? What is the composition of the equilibrium gas phase? e. (10 points) For Mixture C, calculate the mole fraction of the liquid phase. The easiest way to answer this question may be to apply the "lever rule" after measuring with a ruler the tie line identified in part d
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


