Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Reaction of earthen oxides to form stable carbonates has been suggested as a potential process for sequestering carbon dioxide. For instance, iron (II) oxide
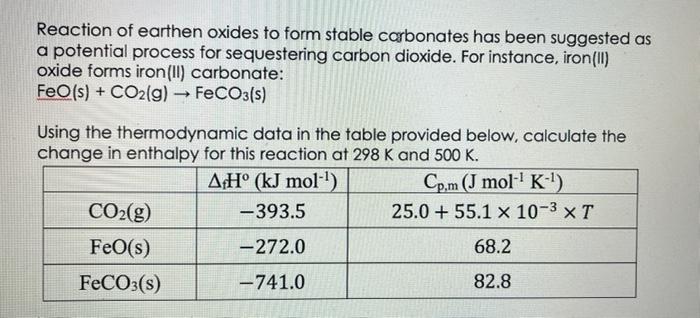
Reaction of earthen oxides to form stable carbonates has been suggested as a potential process for sequestering carbon dioxide. For instance, iron (II) oxide forms iron (II) carbonate: FeO(s) + CO2(g) FeCO3(s) - Using the thermodynamic data in the table provided below, calculate the change in enthalpy for this reaction at 298 K and 500 K. AH (kJ mol-) -393.5 -272.0 -741.0 CO(g) FeO(s) FeCO3(s) Cp.m (J mol- K-) 25.0+ 55.1 x 10-3 x T 68.2 82.8
Step by Step Solution
★★★★★
3.58 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION 8 Reaction of earthen oxides to form sol useful formulas 12 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


