Answered step by step
Verified Expert Solution
Question
1 Approved Answer
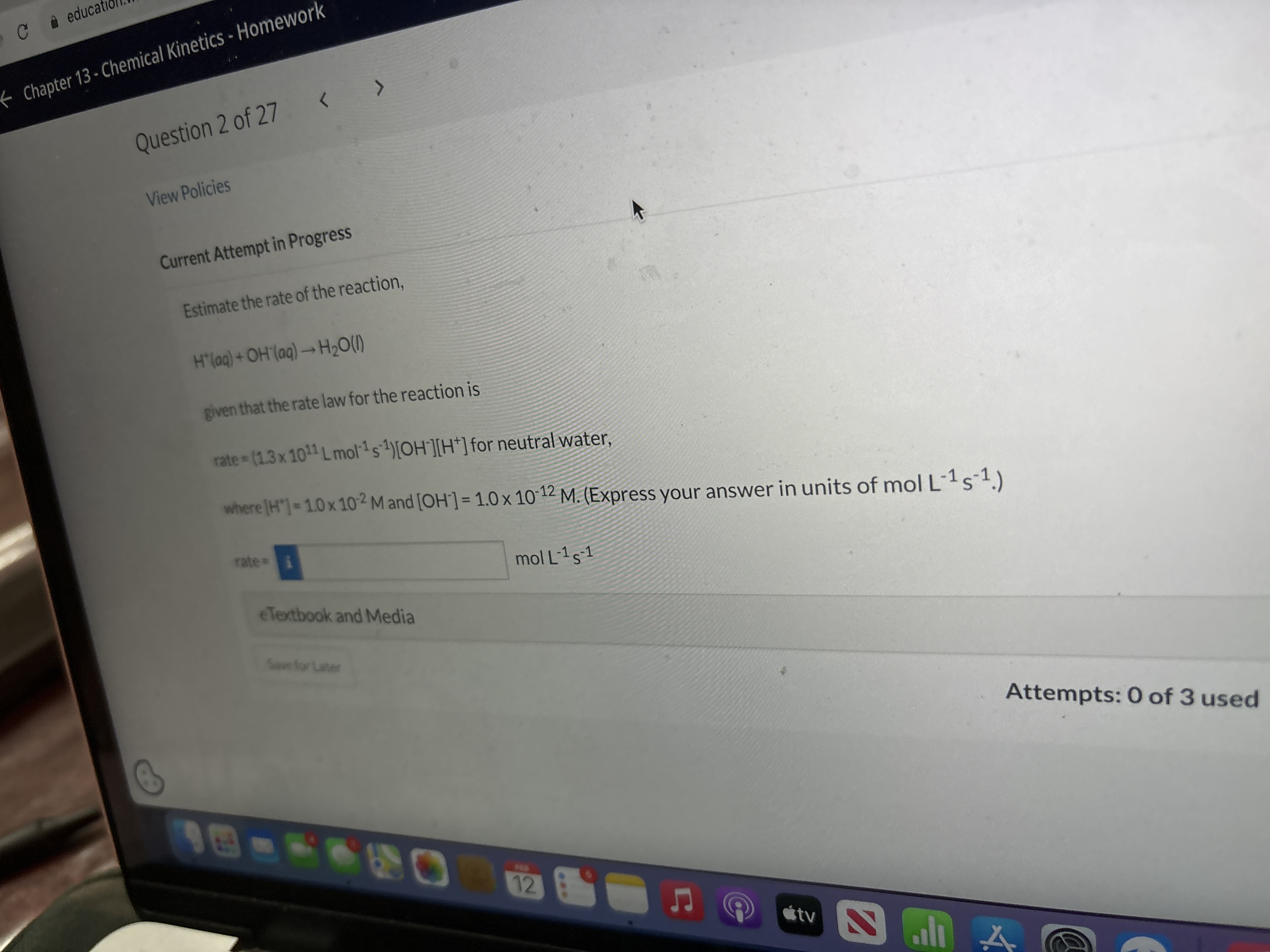
View Policies Current Attempt in Progress Estimate the rate of the reaction, H^(+)(aq)+OH(aq)->H_(2)O(I) given that the rate law for the reaction is rate =(1.3times
View Policies\ Current Attempt in Progress\ Estimate the rate of the reaction,\
H^(+)(aq)+OH(aq)->H_(2)O(I)\ given that the rate law for the reaction is\ rate
=(1.3\\\\times 10^(11)(L)mol^(-1)s^(-1))[OH^(-)][H^(+)]for neutral water,\ where
[H^(+)]=1.0\\\\times 10^(-2)Mand
[OH^(-)]=1.0\\\\times 10^(-12)M. (Express your answer in units of mol L-1
S^(-1).)\
molL^(-1)s^(-1)\ eTextbook and Media
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


