Question
Topic 1 Thermodynamics Objective Questions I (Only one correct option) 1. An ideal gas is allowed to expand from 1 L to 10 L
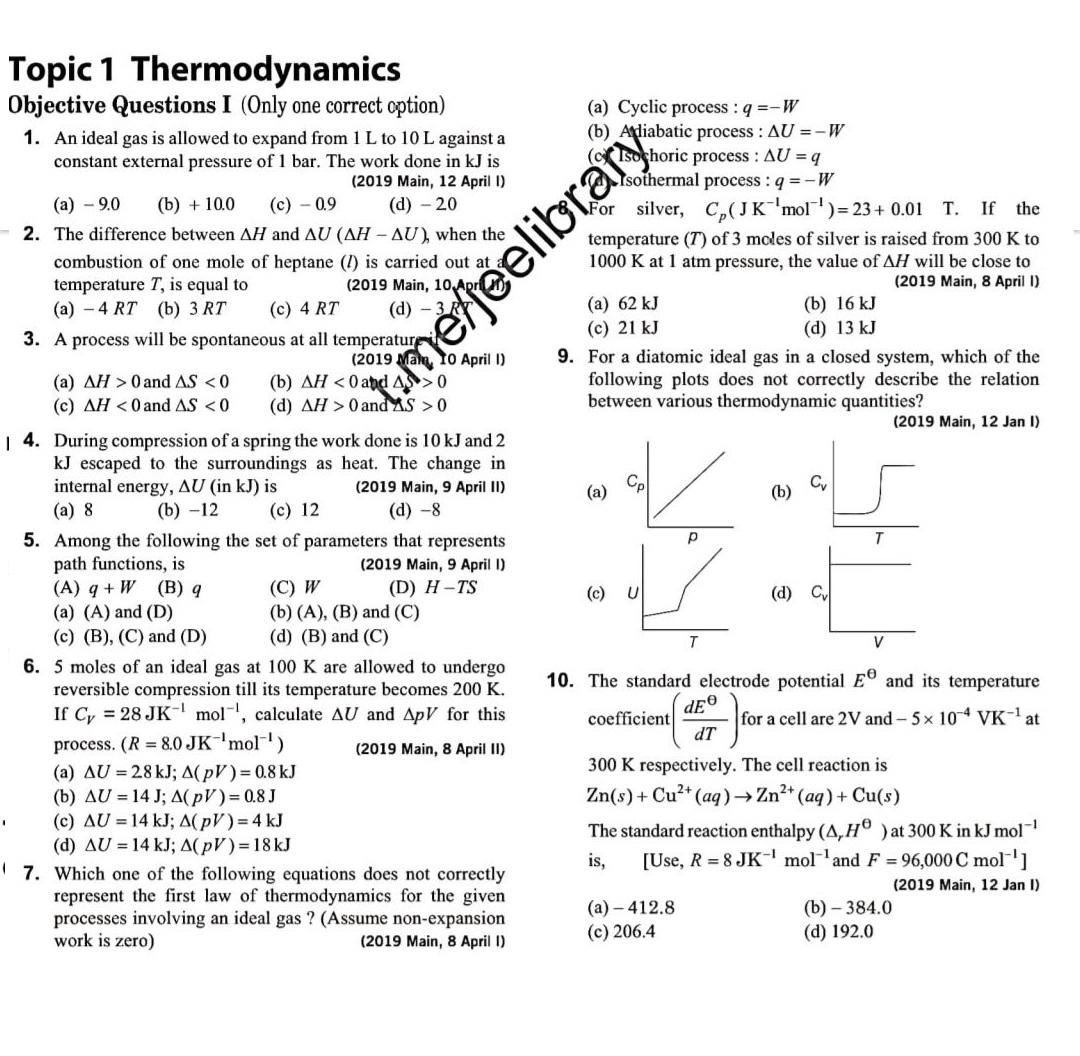
Topic 1 Thermodynamics Objective Questions I (Only one correct option) 1. An ideal gas is allowed to expand from 1 L to 10 L against a constant external pressure of 1 bar. The work done in kJ is (2019 Main, 12 April I) (a) Cyclic process : q =-W (b) Adiabatic process : AU =-W dchoric process : AU = q Isothermal process : q =-W () -9.0 (b) + 10.0 (c) - 0.9 (d) - 20 For silver, C,(JK'mol)= 23+ 0.01 T. If the 2. The difference between AH and AU (AH - AU), when the combustion of one mole of heptane (I) is carried out at temperature T, is equal to () - 4 RT (b) 3 RT temperature (T) of 3 moles of silver is raised from 300 K to 1000 K at 1 atm pressure, the value of AH will be close to (2019 Main, 8 April I) (2019 Main, 10 Apri (a) 62 kJ () 21 kJ (c) 4 RT (b) 16 kJ (d) 13 kJ 3. A process will be spontaneous at all temperatur (a) AH > 0 and AS
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



