Answered step by step
Verified Expert Solution
Question
1 Approved Answer
vol of Walters are 25ml EFFECT OF TEMPERATURE ON SOLID - SOLUTION EQUILIBRIUM Benzoic acid is dissolved in water at three different temperatures and benzoic
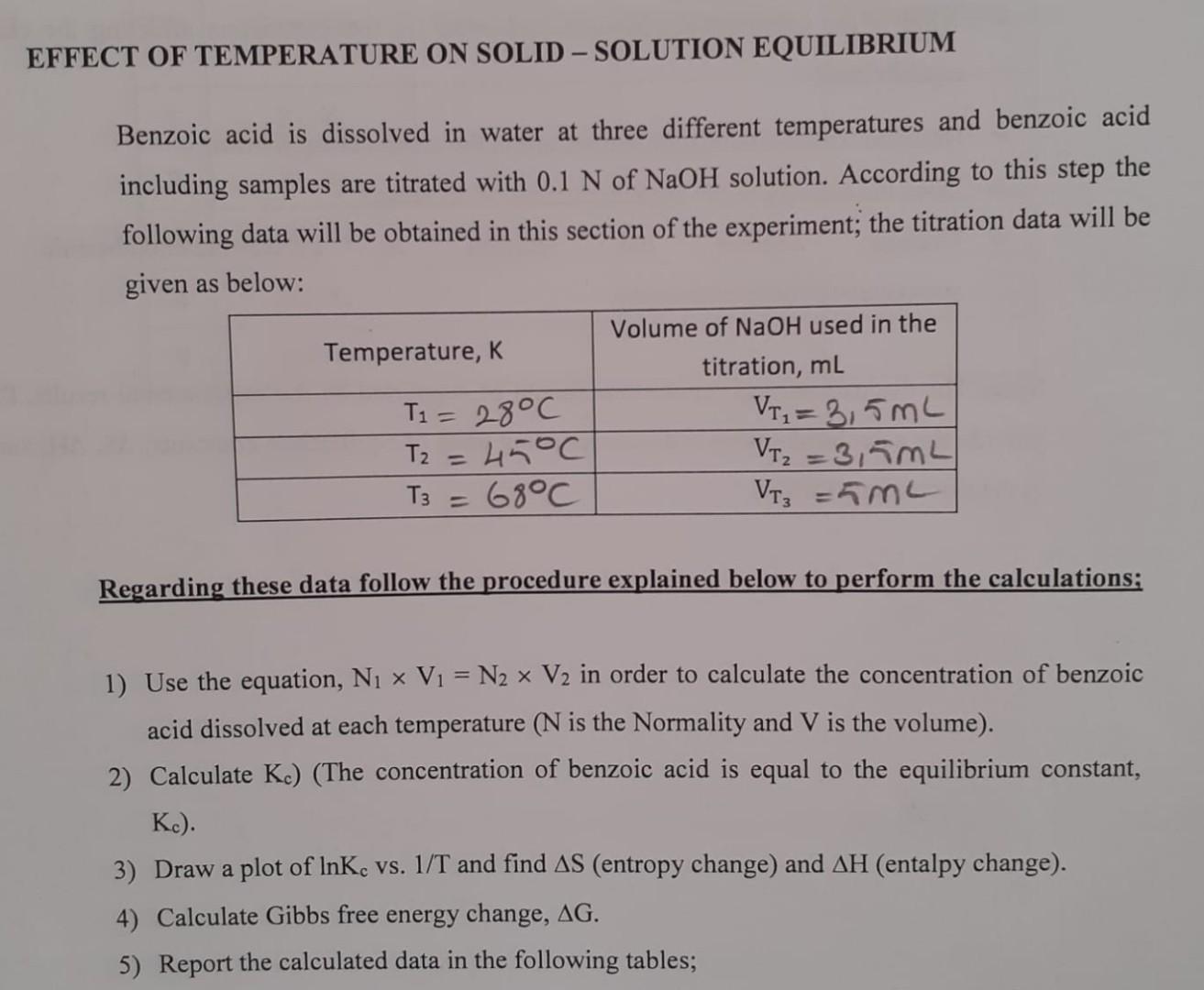
vol of Walters are 25ml
EFFECT OF TEMPERATURE ON SOLID - SOLUTION EQUILIBRIUM Benzoic acid is dissolved in water at three different temperatures and benzoic acid including samples are titrated with 0.1 N of NaOH solution. According to this step the following data will be obtained in this section of the experiment; the titration data will be given as below: Temperature, K T1 = 28C T2 =45C T3 68C Volume of NaOH used in the titration, mL VI = 3,5mL VT =3,5mL VT = 5ML Regarding these data follow the procedure explained below to perform the calculations; 1) Use the equation, Ni x V1 = N2 x V2 in order to calculate the concentration of benzoic acid dissolved at each temperature (N is the Normality and V is the volume). 2) Calculate Kc) (The concentration of benzoic acid is equal to the equilibrium constant, Kc). 3) Draw a plot of InKc vs. 1/T and find AS (entropy change) and AH (entalpy change). 4) Calculate Gibbs free energy change, AG. 5) Report the calculated data in the following tables; EFFECT OF TEMPERATURE ON SOLID - SOLUTION EQUILIBRIUM Benzoic acid is dissolved in water at three different temperatures and benzoic acid including samples are titrated with 0.1 N of NaOH solution. According to this step the following data will be obtained in this section of the experiment; the titration data will be given as below: Temperature, K T1 = 28C T2 =45C T3 68C Volume of NaOH used in the titration, mL VI = 3,5mL VT =3,5mL VT = 5ML Regarding these data follow the procedure explained below to perform the calculations; 1) Use the equation, Ni x V1 = N2 x V2 in order to calculate the concentration of benzoic acid dissolved at each temperature (N is the Normality and V is the volume). 2) Calculate Kc) (The concentration of benzoic acid is equal to the equilibrium constant, Kc). 3) Draw a plot of InKc vs. 1/T and find AS (entropy change) and AH (entalpy change). 4) Calculate Gibbs free energy change, AG. 5) Report the calculated data in the following tablesStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


