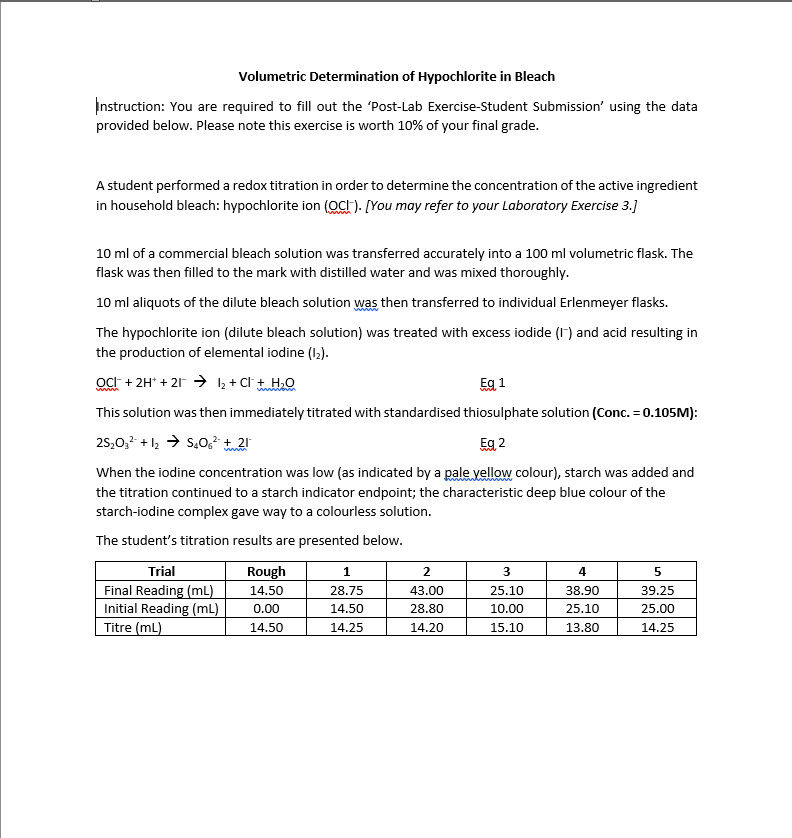

Volumetric Determination of Hypochlorite in Bleach Instruction: You are required to fill out the Post-Lab Exercise-Student Submission' using the data provided below. Please note this exercise is worth 10% of your final grade. A student performed a redox titration in order to determine the concentration of the active ingredient in household bleach: hypochlorite ion (CH). [You may refer to your Laboratory Exercise 3.] 10 ml of a commercial bleach solution was transferred accurately into a 100 ml volumetric flask. The flask was then filled to the mark with distilled water and was mixed thoroughly. 10 ml aliquots of the dilute bleach solution was then transferred to individual Erlenmeyer flasks. The hypochlorite ion (dilute bleach solution) was treated with excess iodide (1) and acid resulting in the production of elemental iodine (12). Oct + 2H+ + 27 + 12 + C + H2O Eg 1 This solution was then immediately titrated with standardised thiosulphate solution (Conc. = 0.105M): 28,0,- + 12 + 5.0+ 21 When the iodine concentration was low (as indicated by a pale yellow colour), starch was added and the titration continued to a starch indicator endpoint; the characteristic deep blue colour of the starch-iodine complex gave way to a colourless solution. The student's titration results are presented below. Eg 2 1 2 3 4 5 Trial Final Reading (mL). Initial Reading (mL) Titre (ml) Rough 14.50 0.00 14.50 28.75 14.50 14.25 43.00 28.80 14.20 25.10 10.00 15.10 38.90 25.10 13.80 39.25 25.00 14.25 Instruction: Fill out the data sheet below using data provided. You should provide calculations on a subsequent page. Concentration of the primary standard potassium iodate = M 1. Average titre of Na S,O3 = mL 2. Number of moles of S2032 required for titration = mol 3. Number of moles of 12 produced in titration = mol (Hint: Eq 2) 4. Number of moles of Och ion present in titrated diluted bleach solution = mol 5. Concentration of Och in diluted bleach solutions M 6. Concentration of Oct in original bleach sample = 7. Mass of Naoci in original bleach sample = M g Assuming density of original bleach is 1.08 g/ml: 8. Mass of original bleach sample used = 8 9. %by mass of NaCl in commercial bleach = % 10. If the label on the bleach bottle claims, "Active ingredient: Sodium hypochlorite 6%", could a certifying analytical chemist accept the claim on the bottle if they work with an acceptance range of +5% of the claim? Why








