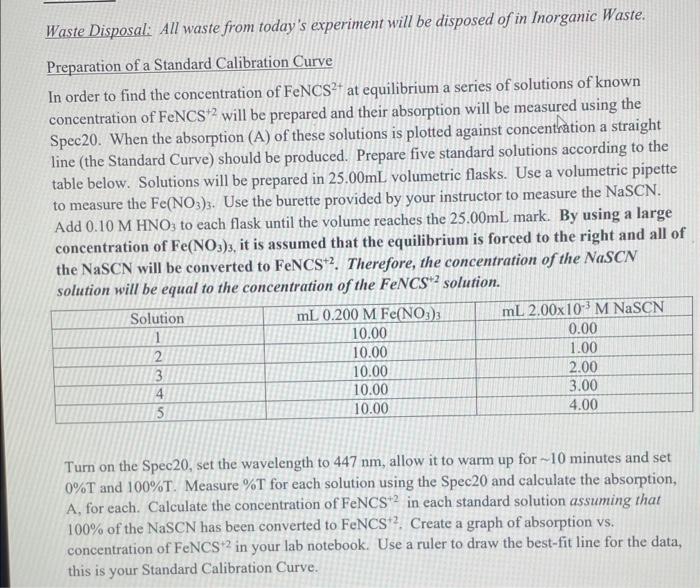
Waste Disposal: All waste from today's experiment will be disposed of in Inorganic Waste. Preparation of a Standard Calibration Curve In order to find the concentration of FeNCS2+ at equilibrium a series of solutions of known concentration of FeNCS +2 will be prepared and their absorption will be measured using the Spec20. When the absorption (A) of these solutions is plotted against concenthation a straight line (the Standard Curve) should be produced. Prepare five standard solutions according to the table below. Solutions will be prepared in 25.00mL volumetric flasks. Use a volumetric pipette to measure the Fe(NO3)3. Use the burette provided by your instructor to measure the NaSCN. Add 0.10MHNO3 to each flask until the volume reaches the 25.00mL mark. By using a large concentration of Fe(NO3)3, it is assumed that the equilibrium is forced to the right and all of the NaSCN will be converted to FeNCS+2. Therefore, the concentration of the NaSCN solution will be equal to the concentration of the FeNCS+2 solution. Turn on the Spec20, set the wavelength to 447nm, allow it to warm up for 10 minutes and set 0%T and 100%T. Measure %T for each solution using the Spec20 and calculate the absorption, A, for each. Calculate the concentration of FeNCS +2 in each standard solution assuming that 100% of the NaSCN has been converted to FeNCS +2. Create a graph of absorption vs. concentration of FeNCS+2 in your lab notebook. Use a ruler to draw the best-fit line for the data, this is your Standard Calibration Curve. Finding Ke Prepare the equilibrium solution by adding 5.00mL of 2.00103MFeNON3)3 and 5.00mL of 2.00103MNaSCN to a 25.00mL volumetric flask. Dilute to the mark with 0.10MHNO3. Allow the solution to stand for approximately 10 minutes to assure that there is time for equilibrium to be established. Measure the \%T of the solution and calculate absorption. Using the Standard Calibration Curve, find the concentration of FeNCS +2 at equilibrium. Calculate the initial concentrations of the reactants and create an ICE table in your lab notebook to find Kc. the percent transmittance (%T). To calculate the amount of light absorbed by the sample (A) from %T the following relationship is used, A=2log(%T) Setting 0%T (Note: Be sure the instrument has been switched on and warmed up for at least 15 minutes before setting 0%T and 100%T ) The equilibrium constant expression for this reaction is: Kc=[Fe3+(aq)][SCN(aq)][FeNCS2(aq)] %T of Solution 1:71.4 % T of Solution 2:27.6 % T of Solution 3: 14.6 \% T of Solution 4: 4.6 \% T of Solution 5: 1.8 \% T of Unknown Solution: 47.2