Answered step by step
Verified Expert Solution
Question
1 Approved Answer
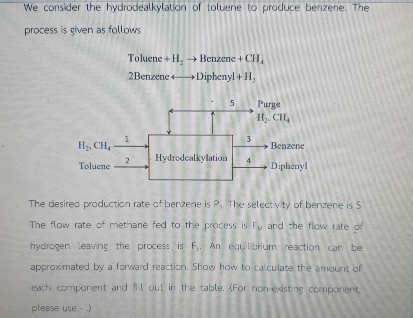
We consider the hydrodealkylation of toluene to produce benzene. The process is given as follows Toluene + H 2 Benzene + C H 4 2
We consider the hydrodealkylation of toluene to produce benzene. The process is given as follows
Toluene Benzene
Benzene longleftrightarrow Diphenyl
The desired production rate ct berzene is The selectivity of benzene is The flow rate of methane fed to the proress is and the flow rate of hydrogen leaving the process is equlturium reaction car be approximated by a formard reaction. Show how to calculate the amount of each comparent and fill out in the table ifor nonexisting cemponent please use
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


