Answered step by step
Verified Expert Solution
Question
1 Approved Answer
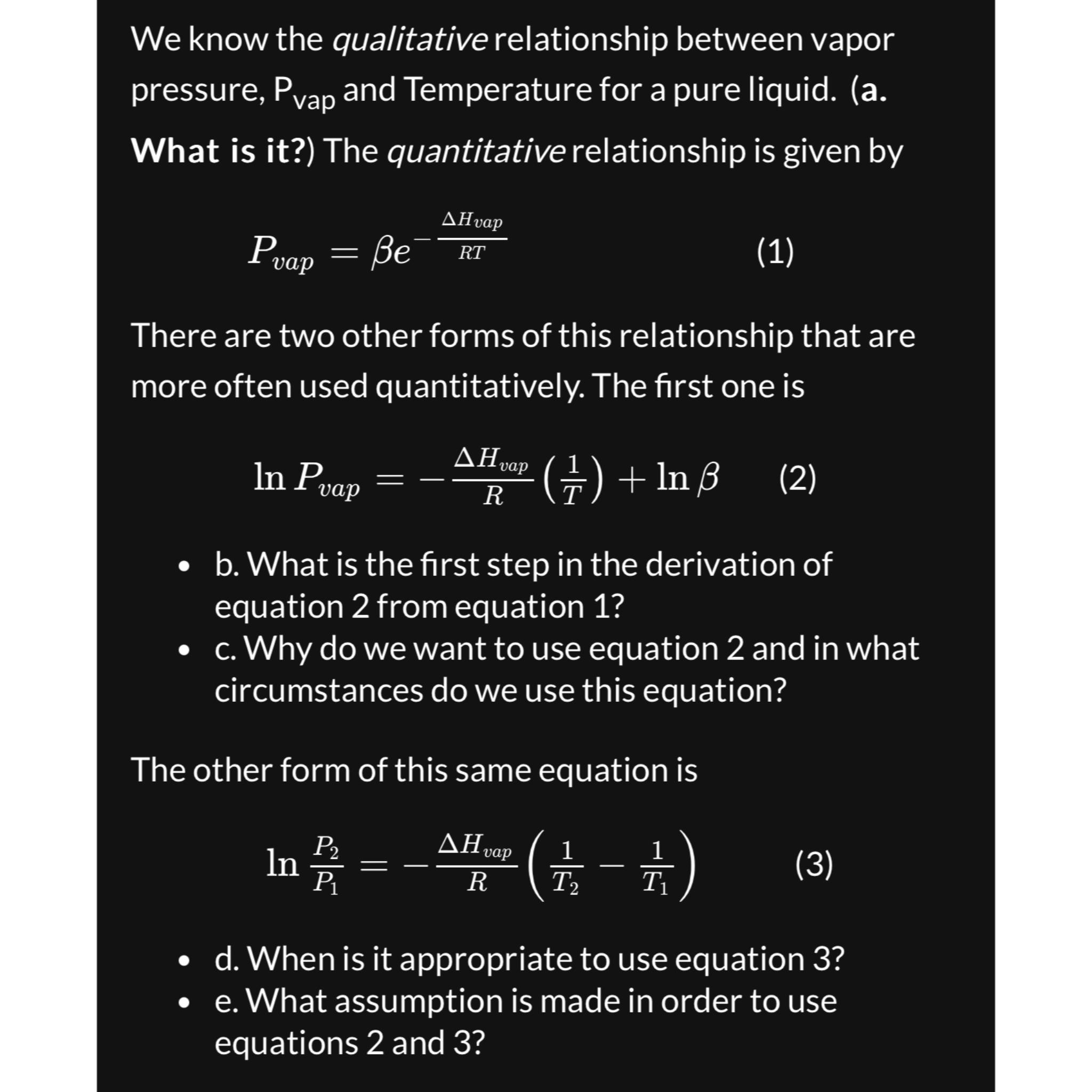
We know the qualitative relationship between vapor pressure, P v a p and Temperature for a pure liquid. ( a . What is it ?
We know the qualitative relationship between vapor pressure, and Temperature for a pure liquid. a What is it The quantitative relationship is given by
There are two other forms of this relationship that are more often used quantitatively. The first one is
b What is the first step in the derivation of equation from equation
c Why do we want to use equation and in what circumstances do we use this equation?
The other form of this same equation is
d When is it appropriate to use equation
e What assumption is made in order to use equations and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


