Answered step by step
Verified Expert Solution
Question
1 Approved Answer
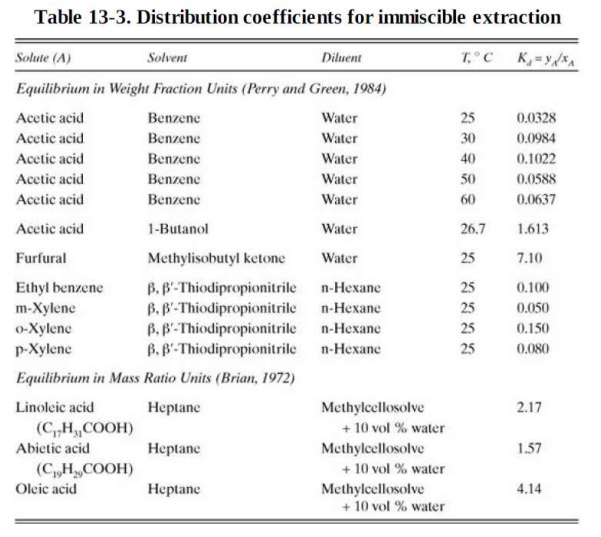
We plan to recover acetic acid from water using 1-butanol as the solvent. Operation is at 26.7C. The feed flow rate is 10.0 kmol/h of
We plan to recover acetic acid from water using 1-butanol as the solvent. Operation is at 26.7C. The feed flow rate is 10.0 kmol/h of an aqueous solution that contains 0.0046 mole frac acetic acid. The entering solvent is pure and flows at 5.0 kmol/h. This operation will be done with three mixer-settlers arranged as a countercurrent cascade. Each mixer-settler can be assumed to be an equilibrium stage. Equilibrium data are available in Table 13-3. Find the exiting raffinate and extract mole fractions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


