Answered step by step
Verified Expert Solution
Question
1 Approved Answer
We want to design a continuous fractionation distillation column to separate a methanol - water mixture. 1 9 , 8 5 0 kg / h
We want to design a continuous fractionation distillation column to separate a methanolwater mixture. kgh are fed, containing methanol and water by weight in the distillate product kmolh is obtained and in the residues they contain water by weight A reflux ratio of moles per mole of product will be used. The molar latent heats of boiling for methanol and water are kJmol and kJmol Assuming that this mixture forms an ideal system, use the relative volatility, for the relevant calculations, at deg C
The feed is saturated steam at the dew point
a Calculate the moles of distilled and residual products per hour.
b Determine the number of ideal plates and the position of the feed plate
c If water steam at an absolute pressure of kPa is used for heating, what amount of steam is required per hour, neglecting heat losses and assuming that the reflux is a saturated liquid?
d If the cooling water enters the condenser at deg C and leaves at deg C what amount of cooling water will be needed?
e What is the minimum reflux ratio?
f What is the minimum number of ideal plates?
g What is the number of real plates with an efficiency of
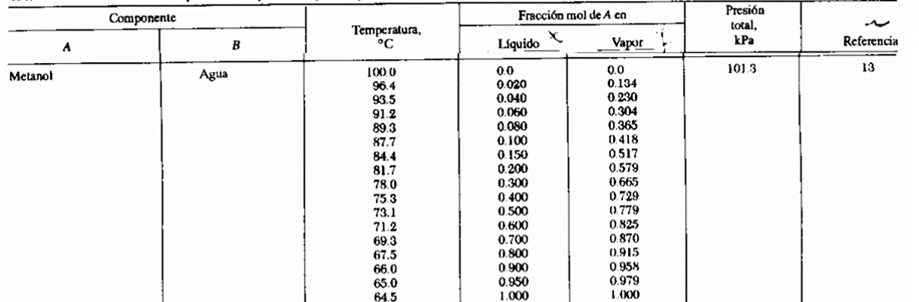
We have the following equilibrium data for the methanolwater system
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


