Question
We want to increase the temperature of the air contained in a closed bottle. The initial temperature and pressure of the air inside the
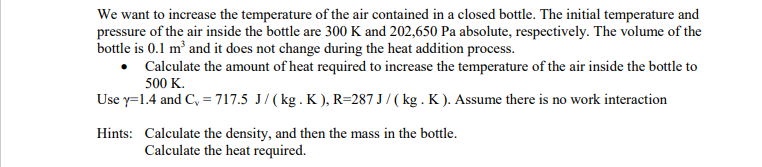
We want to increase the temperature of the air contained in a closed bottle. The initial temperature and pressure of the air inside the bottle are 300 K and 202,650 Pa absolute, respectively. The volume of the bottle is 0.1 m and it does not change during the heat addition process. Calculate the amount of heat required to increase the temperature of the air inside the bottle to 500 K. Use y=1.4 and C = 717.5 J/(kg. K), R=287 J/(kg. K). Assume there is no work interaction Hints: Calculate the density, and then the mass in the bottle. Calculate the heat required.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
First we need to calculate the density ofonsidering that the initial pre...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



