Answered step by step
Verified Expert Solution
Question
1 Approved Answer
We will produce 4 7 , 0 0 0 tons of 9 9 . 5 % purity Phthalic Anhydride. I want you to calculate the
We will produce tons of purity Phthalic Anhydride. I want you to calculate the relative volatility using Antoine coefficients and perform a mass balance calculation from bo FCttom to top. The operating temperature for distillation is degrees Celsius, for distillation it is degrees Celsius, and both operate at ATM pressure. The reactor operates at degrees Celsius and ATM pressure. We assume that the column efficiencies are And we are assuming that oxylene is entering the system at a rate of kmolhour Making products and inputs whose energy balances will be made and whose metal values or cp values will be found to be done for every equipment with heat exchange
Where are pumps used in the system?
Pump and compressor calculation
Where did we use the pump and what source did we use?
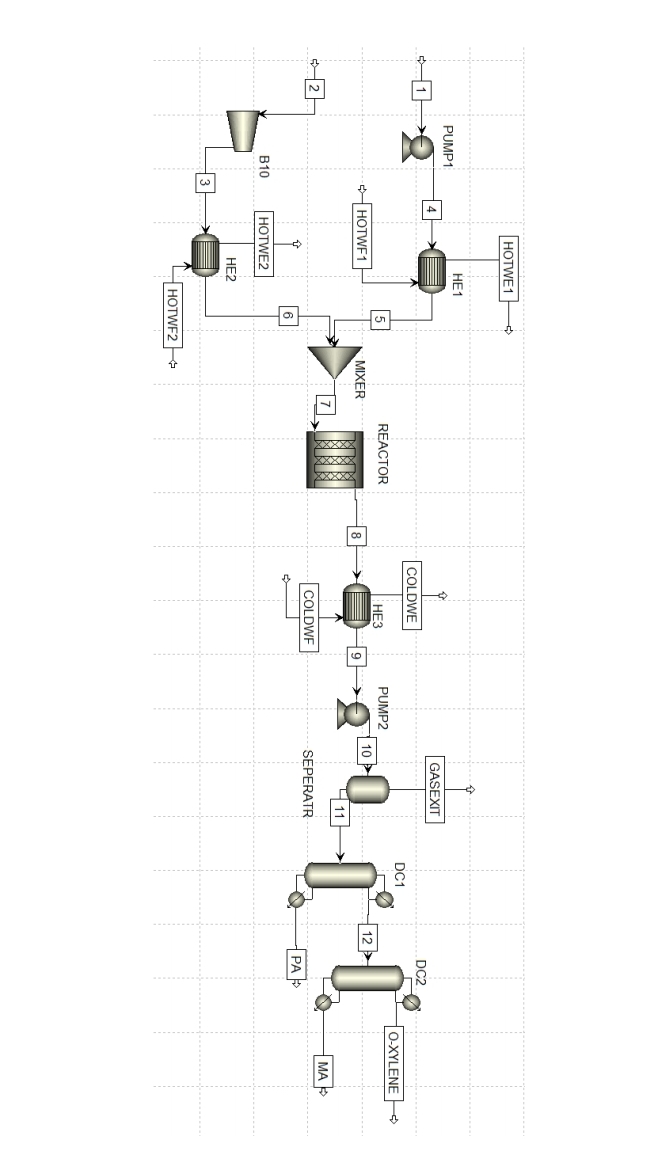
Centrifuge or npe interpretation Phthalic anhydride production is done in several steps. For convenience, oxylene and
The air mixture is preheated. In the process, Oxylene flow at C and atm pressure
It enters the pump. The current fed to the pump is increased to atm pressure. coming out of the pump
The current at C is heated with water at C in the st Heat Exchanger, reaching C
is raised. Air flow inlet conditions are as follows; Fresh air flow is C and atm.
The air flow is increased to atm in the compressor. Current coming out of the compressor Heat
It is raised to C with water at C in the exchanger. Then this current Heat
It is mixed with the stream coming out of the exchanger and fed to the reactor.
Phthalic anhydride, byproduct maleic anhydride and combustion products to be produced in the reactor; It is formed as a result of the oxidation to which oxylene is exposed. Oxidized oxylene feed
and air forms phthalic anhydride. Pure phthalic anhydride at the reactor exit is filled with excess oxygen.
It reacts to form water and carbon dioxide. At the same time, oxylene interacts with oxygen
reacts and releases maleic anhydride as a byproduct, which is a form of maleic anhydride.
Some of it reacts with excess oxygen and forms water and carbon dioxide again.
The hot stream coming out of the reactor is in the gas phase in the rd Heat Exchanger, with C water supply.
It is cooled to C The cooled stream is given to the condenser. Here HO O N and CO
are separated. The remaining product containing Oxylene, FA MA is passed through the nd pump and the pressure is increased to atm.
is dropped. Maleic anhydride as the light key component in the distillation column, heavy
Phthalic anhydride was determined as the key component The one with the highest boiling point
phthalic anhydride, from the bottom of the column; oxylene and maleic have the lowest boiling points
Anhydrite is taken from the top of the column. The main product, phthalic anhydride is in crude FA tanks.
is stored. The oxylene and maleic anhydride stream coming to the nd distillation column is boiling.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


