Answered step by step
Verified Expert Solution
Question
1 Approved Answer
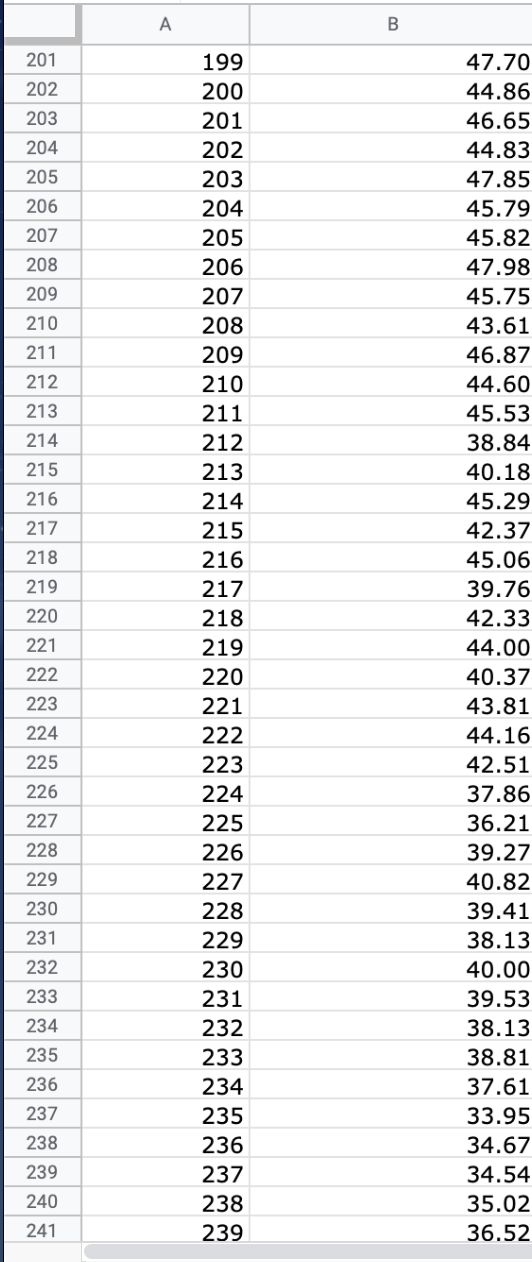
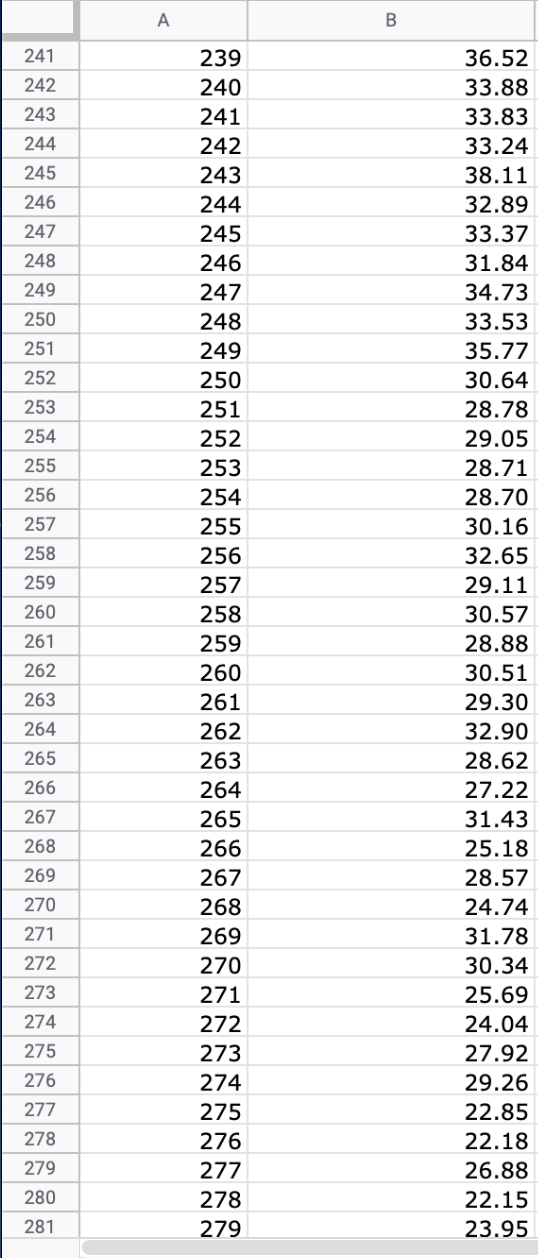
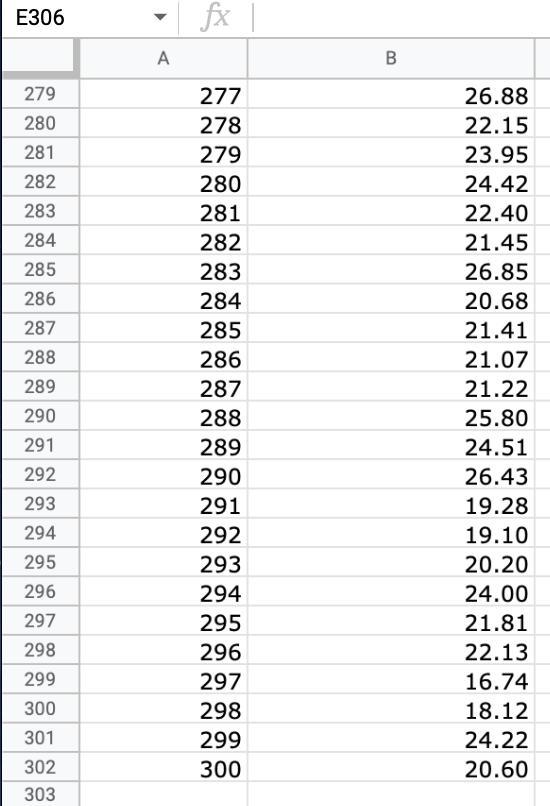
We will use the atmospheric pressure data set from Exercise #1. Create a table in which you report the average atmospheric pressure with its uncertainty
We will use the atmospheric pressure data set from Exercise #1. Create a table in which you report the average atmospheric pressure with its uncertainty (using the proper number of significant figures) five different times. This table should report the pressure using the uncertainties calculated at 68%, 75%, 90%, 95% and 99% confidence limits. Remember that all reported numbers must have units and an appropriate number of significant figures.

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


