Question
We wish to absorb ammonia from an air stream using water at 0C and a total pressure of 1.30 atm. The entering water stream is
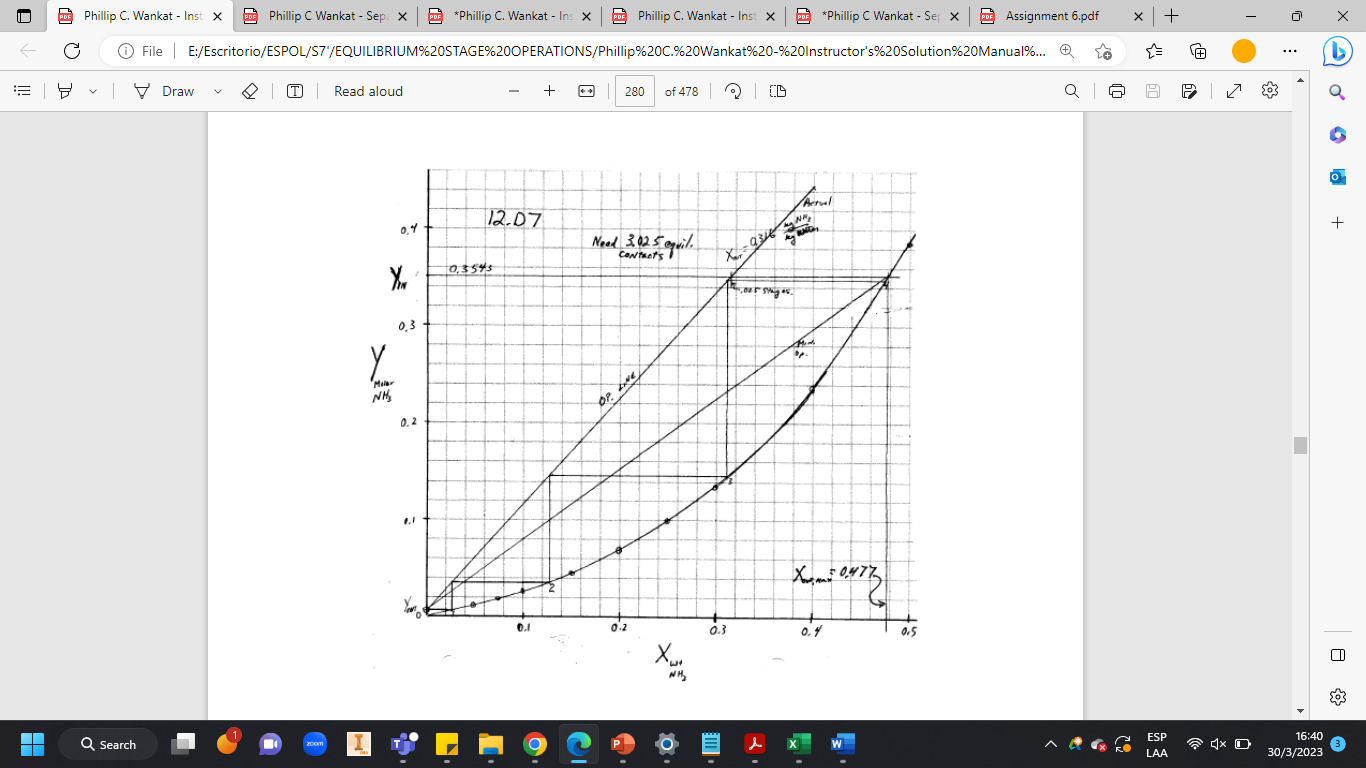
We wish to absorb ammonia from an air stream using water at 0C and a total pressure of 1.30 atm. The entering water stream is pure water. The entering vapor is 17.2 wt % ammonia. We desire to recover 98% of the ammonia in the water outlet stream. The total gas flow rate is 1050 kg/h. We want to use a solvent rate that is 1.5 times the minimum solvent rate. Assume that temperature is constant at 0C, water is nonvolatile, and air does not dissolve in water. Equilibrium data are available in Table 12-3. Find Lmin , L, and N.
Note: I know the solution is on the book, what I don't understand is, where do you get the 0.477. How did you calculate that number? Also the explain the mass balance, why you divide by 17?. The curvy line comes from the x and y, why the book solution its not that accurate with the numbers? Please, God bless you
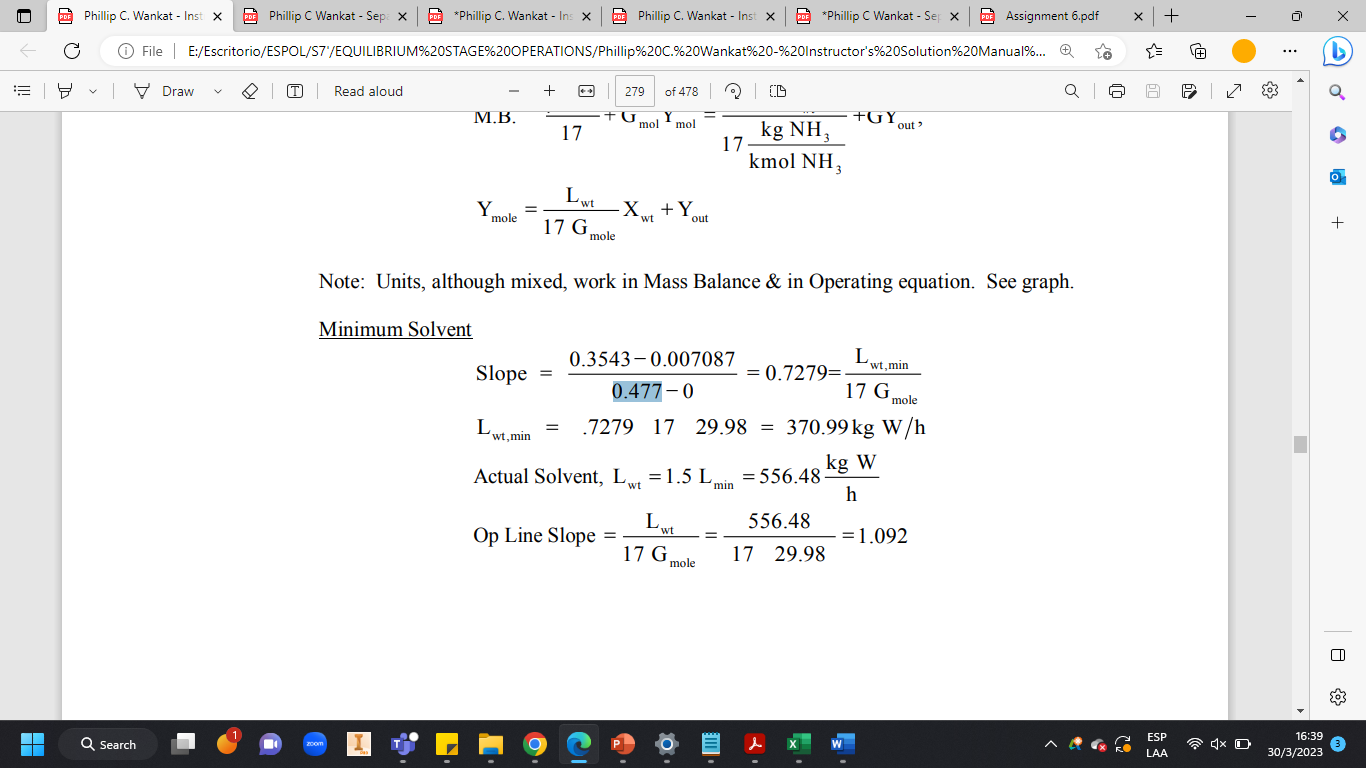
 M.B.17+GmolYmol=17kmolNH3kgNH+KYout,Ymole=17GmoleLwtXwt+Yout Note: Units, although mixed, work in Mass Balance & in Operating equation. See graph. Minimum Solvent Slope=0.47700.35430.007087=0.7279=17GmoleLwt,minLwt,min=.72791729.98=370.99kgW/hActualSolvent,Lwt=1.5Lmin=556.48hkgWOpLineSlope=17GmoleLwt=1729.98556.48=1.092
M.B.17+GmolYmol=17kmolNH3kgNH+KYout,Ymole=17GmoleLwtXwt+Yout Note: Units, although mixed, work in Mass Balance & in Operating equation. See graph. Minimum Solvent Slope=0.47700.35430.007087=0.7279=17GmoleLwt,minLwt,min=.72791729.98=370.99kgW/hActualSolvent,Lwt=1.5Lmin=556.48hkgWOpLineSlope=17GmoleLwt=1729.98556.48=1.092 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


