Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Weak aqueous Sulphuric Acid ( 7 3 % w w is to be concentrated to 9 3 % w w acid by spraying into hot
Weak aqueous Sulphuric Acid is to be concentrated to acid by spraying into hot flue gas at bar pressure so that the excess water evaporates. The flue gas is obtained by the complete combustion, with the stoichiometric amount of air, of a fuel gas of overall composition Sketch a diagram of the process and then calculate the mass of fuel gas required per of concentrated acid produced neglecting heat losses from the concentrator. Data for Acid Data for Flue Gas 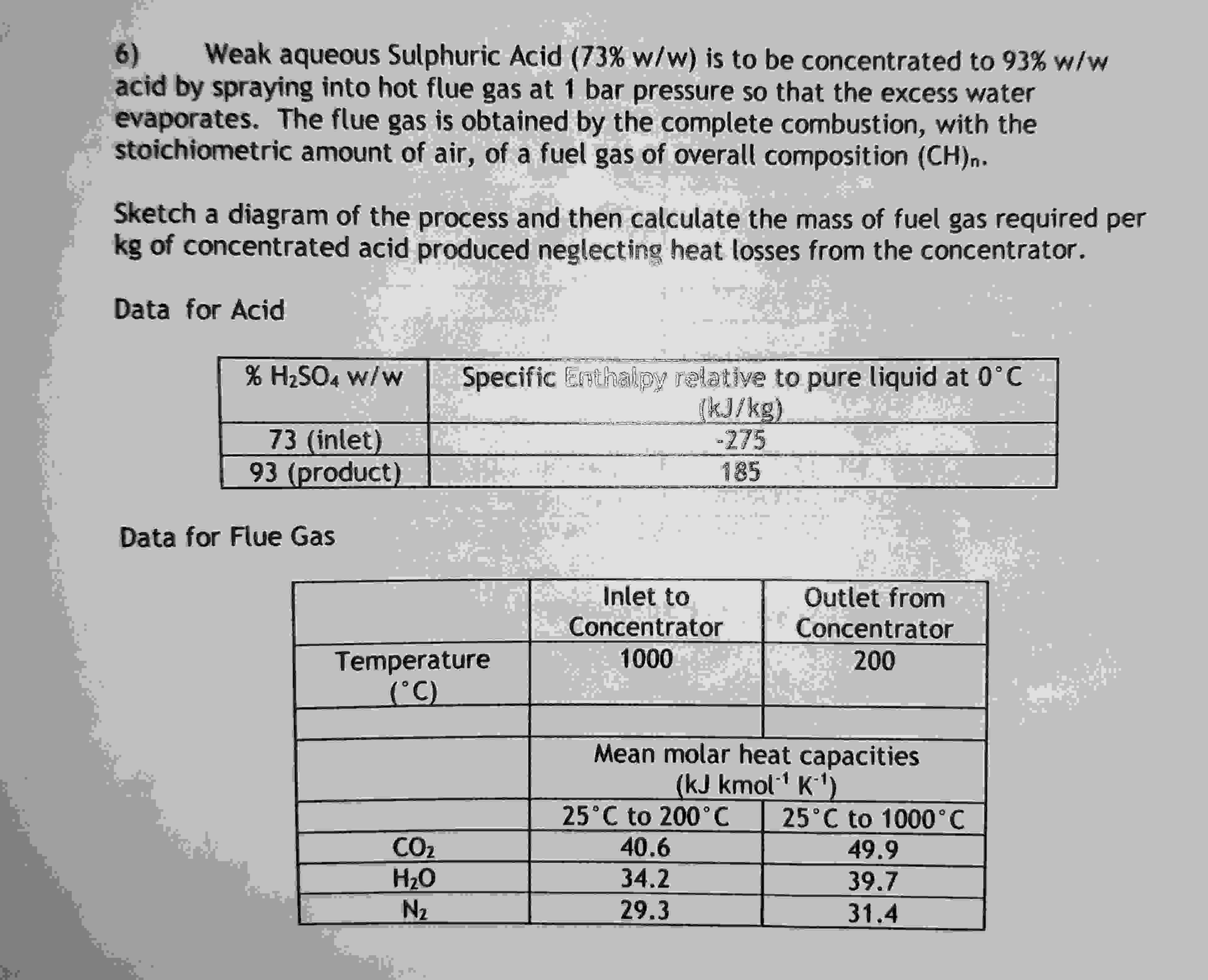
Weak aqueous Sulphuric Acid is to be concentrated to
acid by spraying into hot flue gas at bar pressure so that the excess water
evaporates. The flue gas is obtained by the complete combustion, with the
stoichiometric amount of air, of a fuel gas of overall composition
Sketch a diagram of the process and then calculate the mass of fuel gas required per
of concentrated acid produced neglecting heat losses from the concentrator.
Data for Acid
Data for Flue Gas
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


