Question
Given the initial rate data below, what is the rate law and rate constant for the reaction of iron (II) ion plus oxygen gas
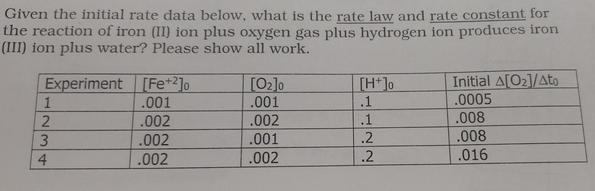
Given the initial rate data below, what is the rate law and rate constant for the reaction of iron (II) ion plus oxygen gas plus hydrogen ion produces iron (III) ion plus water? Please show all work. Experiment 1 23 4 [Fe +2]o .001 .002 .002 .002 [0]0 .001 .002 .001 1.002 [H+]o .1 .1 .2 .2 Initial A[0]/Ato .0005 .008 .008 .016
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
To determine the rate law and rate constant for the given reaction we need to analyze the initial ra...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Law The Ethical Global and E-Commerce Environment
Authors: Jane Mallor, James Barnes, Thomas Bowers, Arlen Langvardt
15th edition
978-0073524986, 73524980, 978-0071317658
Students also viewed these Law questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



