What acts as the dienophile source in this specific experiment?

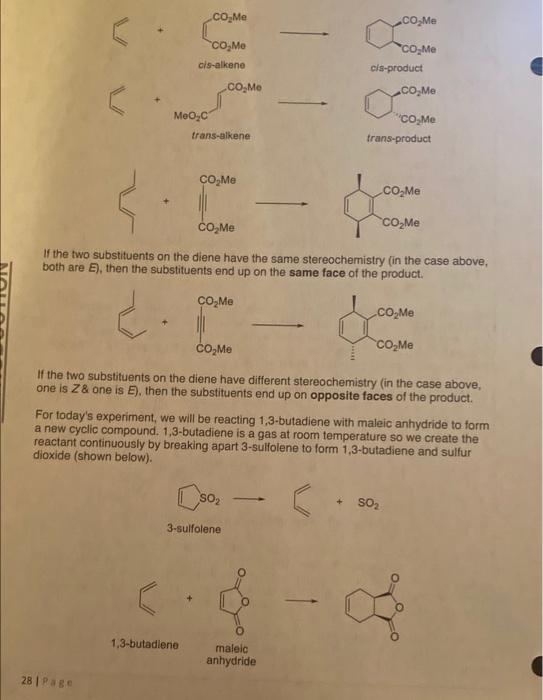
Introduction: You are expected to have prepared a PreLab portion before walking into the lab to perform this experiment (Details on pages 17-20). NEXT WEEK YOUR LAB REPORT SUBMISSION requires the prelab, procedure, results/discussion, conclusion, and completed postiab questions. Diels-Alder reactions are useful as they produce cyclic rings from acyclic precursors. One of the most common uses is to react a 1,3-diene with an alkene to give a 6 membered ring. This reaction forms two new carbon-carbon bonds. The reaction is named for Otto Diels and Kurt Alder, two German chemists who shared the 1950 Nobel Prize in Chemistry for their work on this reaction. This reaction pairs a diene with an alkene, known as a dienophile. The reaction is referred to as a 1,4-cyclo-addition reaction since it forms new bonds on the C1 and C4 of the diene. Figure 1. A sample Diels-Alder reaction. The mechanism for this reaction happens in a concerted fashion, with the bonds breaking and forming simultaneously. The diene must be able to adopt a planar cisoid configuration in order for the reaction to occur. Cisoid, or s-cis, refers to a cis orientation around the single bond (C2-C3). Dienes that are locked in a trans configuration cannot perform this reaction since they can't shift into the proper configuration. Diels-Alder reactions are very stereoselective as the addition reaction occurs in a syn orientation. The cis-dienophile gives cis-substituents on the product, while the transdienophile gives trans-substituents on the product. cis-alkene cla-product trans-alkene trans-product If the two substituents on the diene have the same stereochemistry (in the case above, both are E), then the substituents end up on the same face of the product. If the two substituents on the diene have different stereochemistry (in the case above, one is Z2 one is E ). then the substituents end up on opposite faces of the product. For today's experiment, we will be reacting 1,3-butadiene with maleic anhydride to form a new cyclic compound. 1,3-butadiene is a gas at room temperature so we create the reactant continuously by breaking apart 3-5uliolene to form 1,3-butadiene and sulfur dioxide (shown below). 3-sulfolene 1,3-butadiene maleic anhydride Procedure Note: Before beginning this lab, grind the maleic anhydride in a mortar until it is a fine powder. All your glassware must be DRY for this lab! Place 2.5g of 3-sulfolene, 1.5g of the finely ground maleic anhydride and 1mL of dry xylene into a DRY 25-mL round bottom flask. Add a stir bar to the flask. Attach a condenser to the round bottom flask so that you can heat under reflux (set up the water lines and cool the condenser). Perform this reaction in the HOODIII Noxious sulfur dioxide gas will be produced. Make sure all the windows on your hood are closed while you are performing the reaction. Gently warm and stir the reaction flask until ALL the solid has dissolved. Increase the heat to keep the system under gentle reflux (DO NOT BOIL VIGOROUSLY) for 30 minutes. After refluxing is done, let the flask cool slightly until it is no longer boiling. Add 10mL of xylene to the mixture and reheat it to dissolve any solids. Transfer the hot solution to an Erlenmeyer flask. If solid reappears during this transfer, heat the flask to redissolve the solid. Carefully add petroleum ether to the hot solution until it becomes cloudy (you'll need 5mL or so to get good crystals; it reduces the solubility of the product). Set the solution aside to cool to room temperature. Crystallization will occur. Collect the crystals via vacuum filtration and air dry (or dry on a warm hot plate) before weighing. Determine the melting point and percent yield of your dry product. Show all theoretical yield and percent yield calculations. Waste Disposal: There are three different waste streams for this experiment. 1. Solid Waste goes into the appropriately labeled (Diels-Alder Solids) ziplock bag 2. Used Capillary Tubes must go into the broken glass box. 3. Petroleum Ether and Xylene are simple hydrocarbon and aromatic solvents. Solutions comprising these solvents must be disposed of in the "NonHalogenated" waste containers