Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A frictionless piston-cylinder device initially contains 0.15 kg of water at 200 kPa and 150C. Heat is removed and the system comes to rest
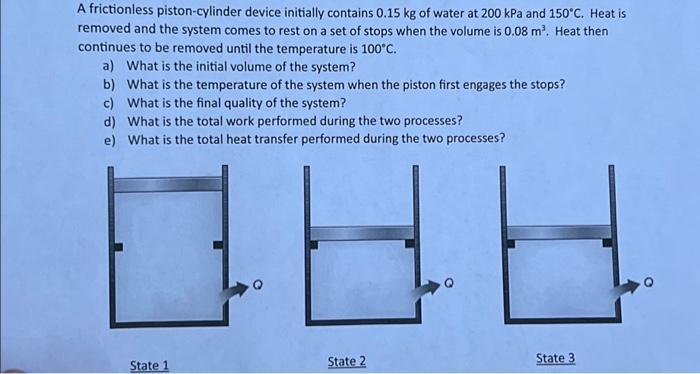
A frictionless piston-cylinder device initially contains 0.15 kg of water at 200 kPa and 150C. Heat is removed and the system comes to rest on a set of stops when the volume is 0.08 m. Heat then continues to be removed until the temperature is 100C. a) What is the initial volume of the system? b) What is the temperature of the system when the piston first engages the stops? c) What is the final quality of the system? d) What is the total work performed during the two processes? e) What is the total heat transfer performed during the two processes? State 1 88 State 2 State 3
Step by Step Solution
★★★★★
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
To solve the given problem we can use the principles of thermodynamics and the properties of water a To find the initial volume of the system we can use the ideal gas law Assuming the water behaves as ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


