Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What are the molar masses of 1H2O, D2O, and T2O? The average mass of O is 15.999 g/mol.What is the molar mass of water on
What are the molar masses of 1H2O, D2O, and T2O? The average mass of O is 15.999 g/mol.What is the molar mass of water on a fictional planet where hydrogen is made up of 33.0% protium, 19.0% deuterium, and 48.0% tritium?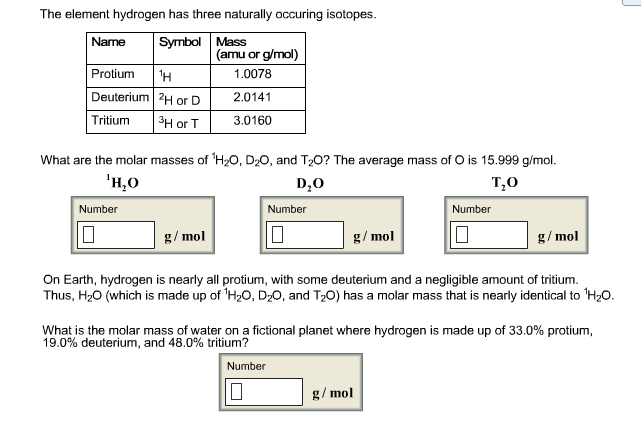
The element hydrogen has three naturally occuring isotopes. Symbol Mass Name (amu or g/mol) Protium H 1.0078 Deuterium 2H or D 2.0141 Tritium 3H or T 3.0160 What are the molar masses of HO, DO, and TO? The average mass of O is 15.999 g/mol. HO DO Number Number g/mol TO Number g/mol g/mol On Earth, hydrogen is nearly all protium, with some deuterium and a negligible amount of tritium. Thus, HO (which is made up of 'HO, DO, and TO) has a molar mass that is nearly identical to HO. What is the molar mass of water on a fictional planet where hydrogen is made up of 33.0% protium, 19.0% deuterium, and 48.0% tritium? Number g/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the molar mass of water on the fictional planet where hydrogen is composed of 330 pr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663de8612bc76_961164.pdf
180 KBs PDF File
663de8612bc76_961164.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


