Question
Methanol (CH3OH) is produced by reacting CO and H2 according to the equation CO+2H2 CHOH Only 15% of the CO entering the reactor is
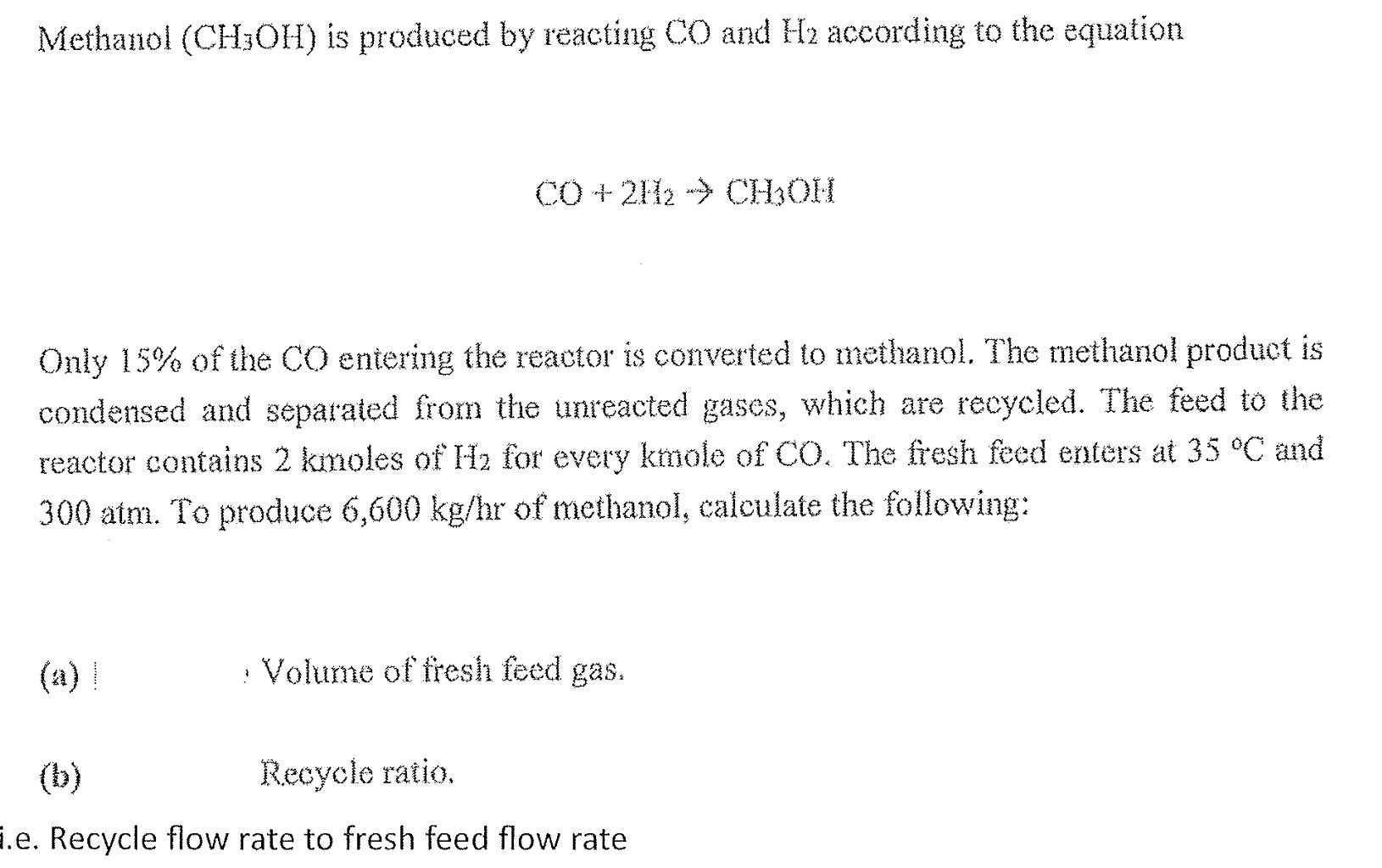
Methanol (CH3OH) is produced by reacting CO and H2 according to the equation CO+2H2 CHOH Only 15% of the CO entering the reactor is converted to methanol. The methanol product is condensed and separated from the unreacted gases, which are recycled. The feed to the reactor contains 2 kmoles of H for every kmole of CO. The fresh feed enters at 35 C and 300 atm. To produce 6,600 kg/hr of methanol, calculate the following: Volume of fresh feed gas. Recycle ratio. i.e. Recycle flow rate to fresh feed flow rate
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Calculating Methanol Production Parameters Given Methanol equation CO 2H2 CH3OH CO conversion 15 Fee...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry Principles And Modern Applications
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
11th Edition
0132931281, 978-0132931281
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



