Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what can you conclude from the following results? (.5 a point was deducted off) What can you conclude from the following results? (Choose all that
what can you conclude from the following results?
What can you conclude from the following results? (Choose all that apply.) Trial Water 1 50 2. 37 3 48 4 39 5 46 Average 44 Salt water 27 22 28 21 25 25 Soapy Rubbing water alcohol 15 30 17 28 13 32 18 35 15 31 16 31 The addition of salt decreases the attractions between water molecules The Intermolecular forces in soapy water are the weakest among the liquids tested here. The addition of soap to water increases the attraction between water molecules. The intermolecular forces between water molecules are the strongest among the liquids tested here. The intermolecular forces between rubbing alcohol molecules are weaker than those between water molecules (.5 a point was deducted off) 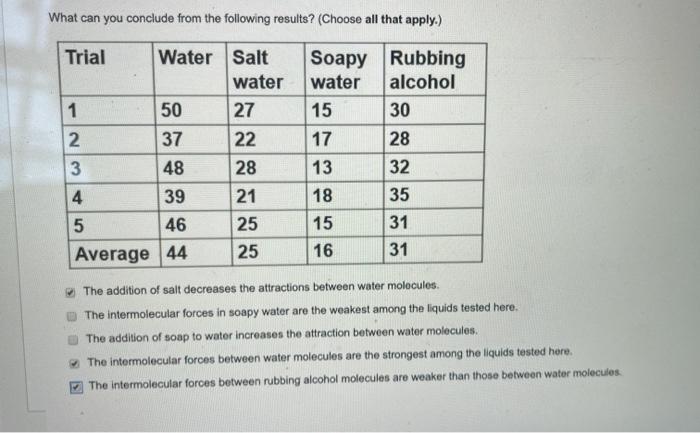

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


