Answered step by step
Verified Expert Solution
Question
1 Approved Answer
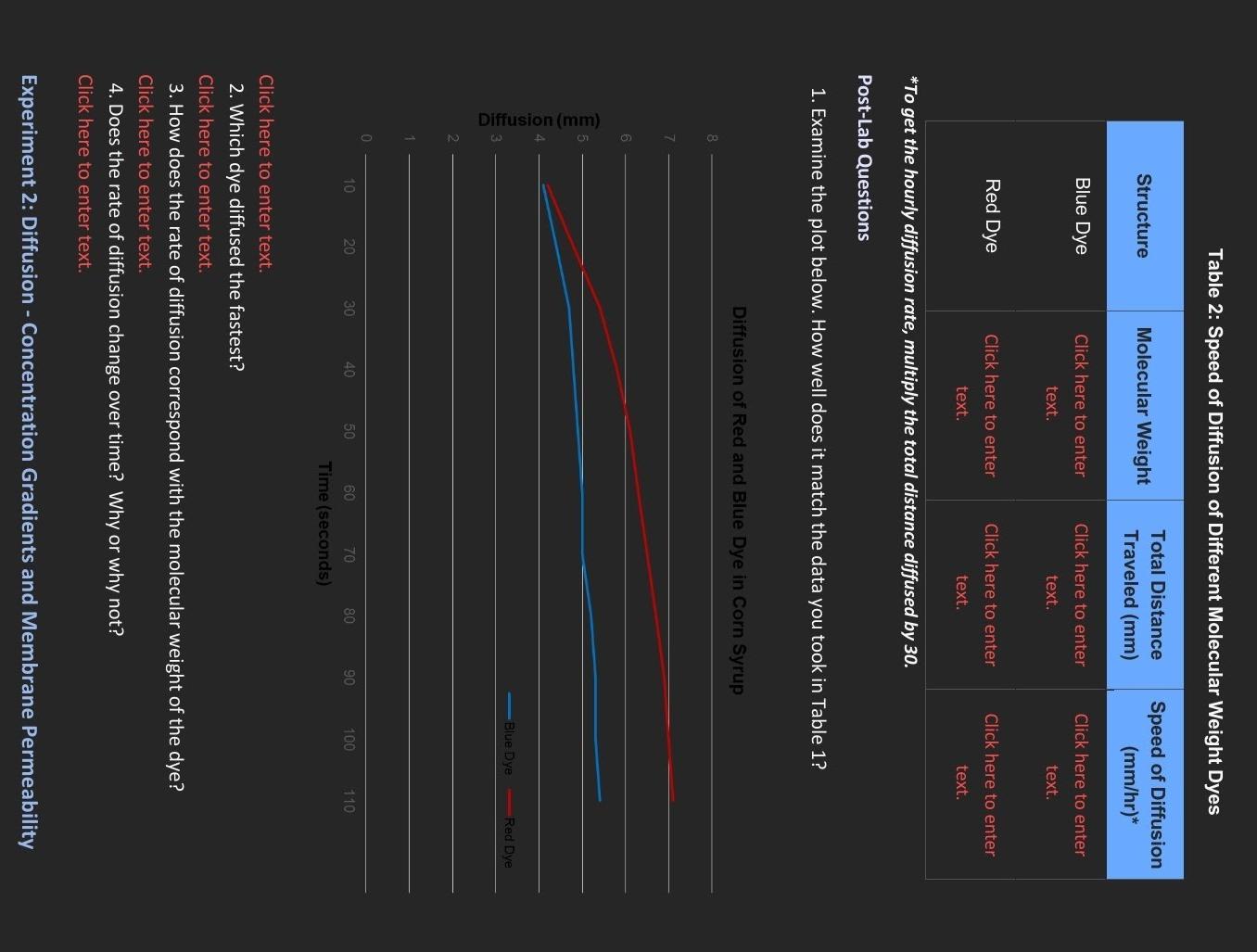
what clarification is needed? Table 2: Speed of Diffusion of Different Molecular Weight Dyes *To get the hourly diffusion rate, multiply the total distance diffused
what clarification is needed?
Table 2: Speed of Diffusion of Different Molecular Weight Dyes *To get the hourly diffusion rate, multiply the total distance diffused by 30. Post-Lab Questions 1. Examine the plot below. How well does it match the data you took in Table 1? Diffusion of Red and Blue Dye in Corn Syrup 8 7 6 Click here to enter text. 2. Which dye diffused the fastest? Click here to enter text. 3. How does the rate of diffusion correspond with the molecular weight of the dye? Click here to enter text. 4. Does the rate of diffusion change over time? Why or why not? Click here to enter text. Experiment 2: Diffusion - Concentration Gradients and Membrane PermeabilityStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


