Question
What comes to mind when you hear the word, thermodynamics? First law of thermodynamics: Energy conservation applied to the system and its surroundings. In
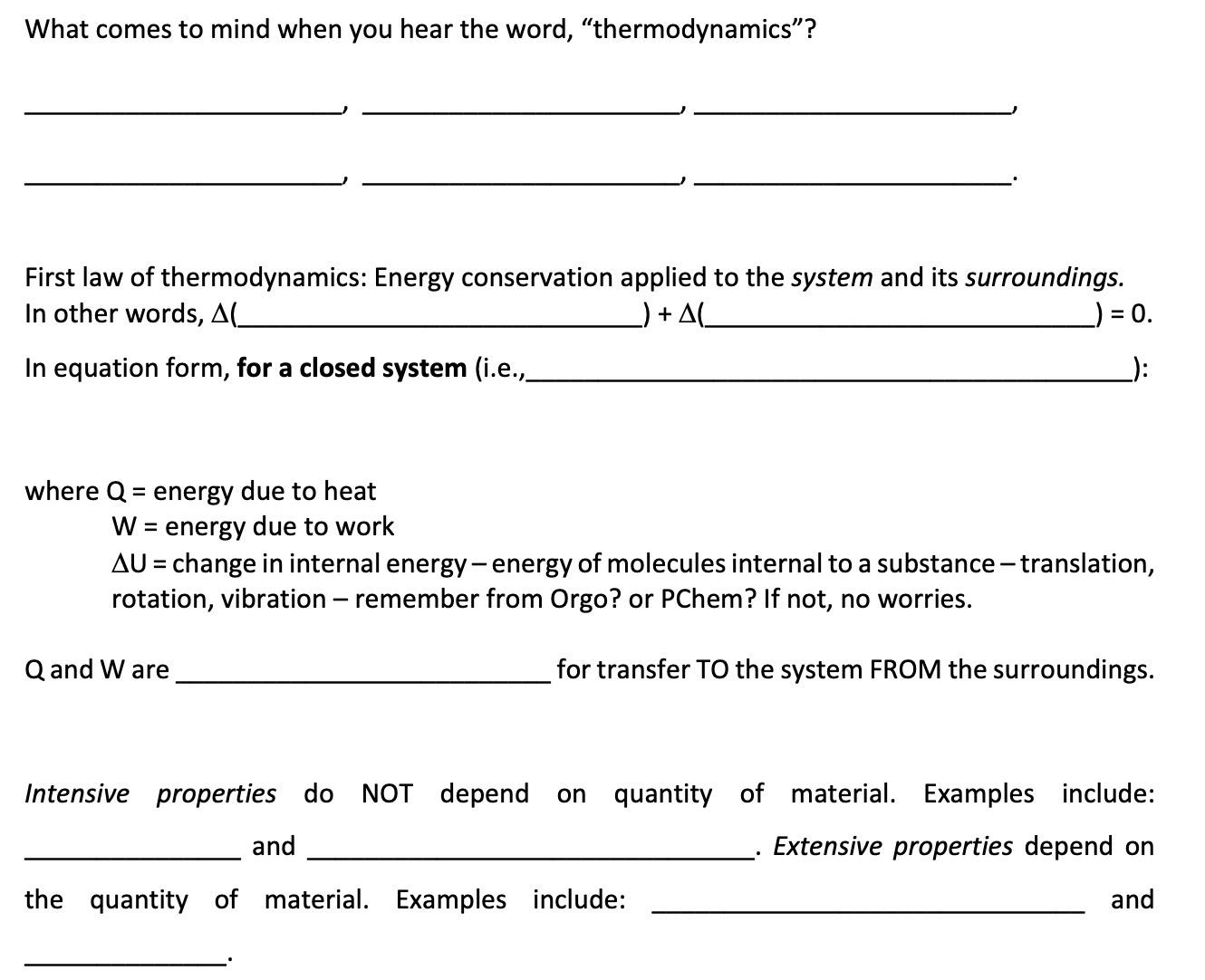
What comes to mind when you hear the word, "thermodynamics"? First law of thermodynamics: Energy conservation applied to the system and its surroundings. In other words, A(_ In equation form, for a closed system (i.e.,_ ) + A(_ ) = 0. where Q = energy due to heat W = energy due to work AU = change in internal energy - energy of molecules internal to a substance - translation, rotation, vibration - remember from Orgo? or PChem? If not, no worries. Q and W are for transfer TO the system FROM the surroundings. Intensive properties do NOT depend on quantity of material. Examples include: and the quantity of material. Examples include: Extensive properties depend on and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of Marketing
Authors: Philip Kotler, Gary Armstrong
14th Edition
132167123, 132997266, 9780132997263, 978-0132167123
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



